In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.

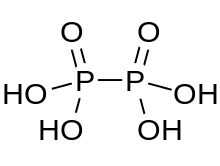
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P−O−P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate and tetrasodium pyrophosphate, among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P−O−P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.

In chemistry tellurate is a compound containing an oxyanion of tellurium where tellurium has an oxidation number of +6. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central tellurium atom.
In chemistry, phosphorus oxoacid is a generic name for any acid whose molecule consists of atoms of phosphorus, oxygen, and hydrogen. There is a potentially infinite number of such compounds. Some of them are unstable and have not been isolated, but the derived anions and organic groups are present in stable salts and esters. The most important ones—in biology, geology, industry, and chemical research—are the phosphoric acids, whose esters and salts are the phosphates.

Calcium pyrophosphate (Ca2P2O7) is a chemical compound, an insoluble calcium salt containing the pyrophosphate anion. There are a number of forms reported: an anhydrous form, a dihydrate, Ca2P2O7·2H2O and a tetrahydrate, Ca2P2O7·4H2O. Deposition of dihydrate crystals in cartilage are responsible for the severe joint pain in cases of calcium pyrophosphate deposition disease (pseudo gout) whose symptoms are similar to those of gout. Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes, because of its insolubility and nonreactivity toward fluoride.

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, SMFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).
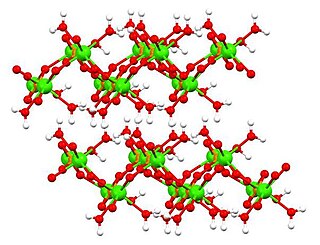
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial.

Dihydrogen phosphate is an inorganic ion with the formula [H2PO4]−. Phosphates occur widely in natural systems.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Monosodium phosphate (MSP), also known as monobasic sodium phosphate and sodium dihydrogen phosphate, is an inorganic compound with the chemical formula NaH2PO4. It is a sodium salt of phosphoric acid. It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4). One of many sodium phosphates, it is a common industrial chemical. The salt exists in an anhydrous form, as well as monohydrate and dihydrate (NaH2PO4·H2O and NaH2PO4·2H2O respectively).
The nickel organic acid salts are organic acid salts of nickel. In many of these the ionised organic acid acts as a ligand.

Potassium diplatinum(II) tetrakispyrophosphite (abbreviated as [Pt2(pop)4]4−) is the inorganic compound with the formula K4[Pt2(HO2POPO2H)4]. It is a water-soluble yellow salt. The compound has a long-lived, strongly luminescent excited state, with an emission maximum at ~510 nm and a lifetime near 10 μs.
Cobalt compounds are chemical compounds formed by cobalt with other elements.