Related Research Articles
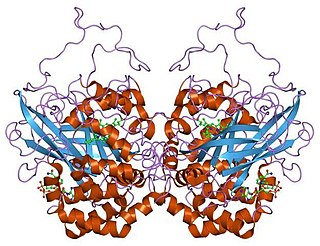
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

Cystic fibrosis (CF) is a rare genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. The hallmark feature of CF is the accumulation of thick mucus in different organs. Long-term issues include difficulty breathing and coughing up mucus as a result of frequent lung infections. Other signs and symptoms may include sinus infections, poor growth, fatty stool, clubbing of the fingers and toes, and infertility in most males. Different people may have different degrees of symptoms.

Heme, or haem, is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
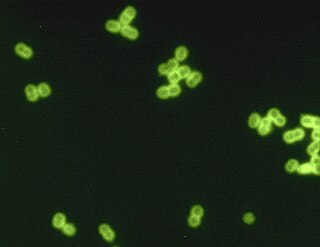
Streptococcus pneumoniae, or pneumococcus, is a Gram-positive, spherical bacteria, alpha-hemolytic member of the genus Streptococcus. They are usually found in pairs (diplococci) and do not form spores and are non motile. As a significant human pathogenic bacterium S. pneumoniae was recognized as a major cause of pneumonia in the late 19th century, and is the subject of many humoral immunity studies.

In chemistry and biology, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (O2), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (O2H), superoxide (O2-), hydroxyl radical (OH.), and singlet oxygen. ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.

Lactoferrin (LF), also known as lactotransferrin (LTF), is a multifunctional protein of the transferrin family. Lactoferrin is a globular glycoprotein with a molecular mass of about 80 kDa that is widely represented in various secretory fluids, such as milk, saliva, tears, and nasal secretions. Lactoferrin is also present in secondary granules of PMNs and is secreted by some acinar cells. Lactoferrin can be purified from milk or produced recombinantly. Human colostrum has the highest concentration, followed by human milk, then cow milk (150 mg/L).

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.

Thiocyanates are salts containing the thiocyanate anion [SCN]−. [SCN]− is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics.

Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the CFTR gene.
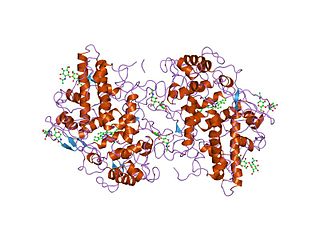
Myeloperoxidase (MPO) is a peroxidase enzyme that in humans is encoded by the MPO gene on chromosome 17. MPO is most abundantly expressed in neutrophils, and produces hypohalous acids to carry out their antimicrobial activity, including hypochlorous acid, the sodium salt of which is the chemical in bleach. It is a lysosomal protein stored in azurophilic granules of the neutrophil and released into the extracellular space during degranulation. Neutrophil myeloperoxidase has a heme pigment, which causes its green color in secretions rich in neutrophils, such as mucus and sputum. The green color contributed to its outdated name verdoperoxidase.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
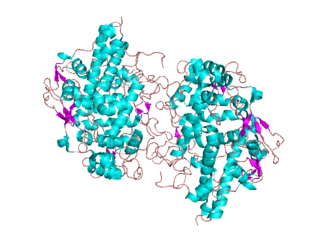
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin.

Dual oxidase 2, also known as DUOX2 or ThOX2, is an enzyme that in humans is encoded by the DUOX2 gene. Dual oxidase is an enzyme that was first identified in the mammalian thyroid gland. In humans, two isoforms are found; hDUOX1 and hDUOX2. The protein location is not exclusive to thyroid tissue; hDUOX1 is prominent in airway epithelial cells and hDUOX2 in the salivary glands and gastrointestinal tract.

Dual oxidase 1, also known as DUOX1 or ThOX1, is an enzyme which in humans is encoded by the DUOX1 gene. DUOX1 was first identified in the mammalian thyroid gland. In humans, two isoforms are found; hDUOX1 and hDUOX2. Human DUOX protein localization is not exclusive to thyroid tissue; hDUOX1 is prominent in airway epithelial cells and hDUOX2 in the salivary glands and gastrointestinal tract.
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment.

Pyocyanin (PCN−) is one of the many toxic compounds produced and secreted by the Gram negative bacterium Pseudomonas aeruginosa. Pyocyanin is a blue secondary metabolite, turning red below pH 4.9, with the ability to oxidise and reduce other molecules and therefore kill microbes competing against P. aeruginosa as well as mammalian cells of the lungs which P. aeruginosa has infected during cystic fibrosis. Since pyocyanin is a zwitterion at blood pH, it is easily able to cross the cell membrane. There are three different states in which pyocyanin can exist: oxidized (blue), monovalently reduced (colourless) or divalently reduced (red). Mitochondria play an important role in the cycling of pyocyanin between its redox states. Due to its redox-active properties, pyocyanin generates reactive oxygen species.
The respiratory tract antimicrobial defense system is a layered defense mechanism which relies on components of both the innate and adaptive immune systems to protect the lungs and the rest of the respiratory tract against inhaled microorganisms.

Lactoperoxidase is a peroxidase enzyme secreted from mammary, salivary and other mucosal glands including the lungs, bronchii and nose that functions as a natural and the first line of defense against bacteria and viruses. Lactoperoxidase is a member of the heme peroxidase family of enzymes. In humans, lactoperoxidase is encoded by the LPO gene.

Eosinophil peroxidase is an enzyme found within the eosinophil granulocytes, innate immune cells of humans and mammals. This oxidoreductase protein is encoded by the gene EPX, expressed within these myeloid cells. EPO shares many similarities with its orthologous peroxidases, myeloperoxidase (MPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO). The protein is concentrated in secretory granules within eosinophils. Eosinophil peroxidase is a heme peroxidase, its activities including the oxidation of halide ions to bacteriocidal reactive oxygen species, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues.

Sujata Sharma is an Indian structural biologist, biophysicist, writer and a professor at the Department of Biophysics of the All India Institute of Medical Sciences, Delhi. She is known for her studies in the fields of protein structure, drug design and drug resistance of bacteria. Her studies have been documented by way of a number of articles and ResearchGate, an online repository of scientific articles has listed 167 of them. She is also the author of the books, "Warriors in White", an autobiographical account of some COVID-19 Warriors at All India Institute of Medical Sciences, Delhi and other leading hospitals of India, including Prof Randeep Guleria, using a combination of modern medicine, astronomy and Vedic astrology, "The Secret of the Red Crystals", an autobiographical account of her days in AIIMS Delhi. and "A Dragonfly's purpose", which is an autobiographical account of her recovery from an autoimmune disease, Guillain Barre Syndrome. The Department of Biotechnology of the Government of India awarded her the National Bioscience Award for Career Development, one of the highest Indian science awards, for her contributions to biosciences, in 2011. She is also a recipient of the Woman Scientist Award of the Biotech Research Society of India and the National Young Woman Bioscientist Award of the Department of Biotechnology which she received in 2006 and 2007 respectively. In 2020, she was awarded the Kalpana Chawla Excellence award, for her contributions in science. This award is instituted in the memory of the first Indian woman astronaut, Kalpana Chawla to go on space missions.
References
- ↑ Furtmüller PG, Zederbauer M, Jantschko W, Helm J, Bogner M, Jakopitsch C, Obinger C (January 2006). "Active site structure and catalytic mechanisms of human peroxidases". Arch. Biochem. Biophys. 445 (2): 199–213. doi:10.1016/j.abb.2005.09.017. PMID 16288970.
- ↑ Al Obaidi AH (July 2007). "Role of airway lactoperoxidase in scavenging of hydrogen peroxide damage in asthma". Ann Thorac Med. 2 (3): 107–10. doi: 10.4103/1817-1737.33698 . PMC 2732085 . PMID 19727356.
- ↑ Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Nauseef WM, Dupuy C, Bánfi B (January 2007). "A Novel Host Defense System of Airways Is Defective in Cystic Fibrosis". Am. J. Respir. Crit. Care Med. 175 (2): 174–83. doi:10.1164/rccm.200607-1029OC. PMC 2720149 . PMID 17082494.
- ↑ Carlsson J, Edlund MB, Hänström L (June 1984). "Bactericidal and cytotoxic effects of hypothiocyanite-hydrogen peroxide mixtures". Infect. Immun. 44 (3): 581–6. doi:10.1128/IAI.44.3.581-586.1984. PMC 263633 . PMID 6724690.
- ↑ Mansson-Rahemtulla B, Pruitt KM, Tenovuo J, Le TM (October 1983). "A mouthrinse which optimizes in vivo generation of hypothiocyanite". J. Dent. Res. 62 (10): 1062–6. doi:10.1177/00220345830620101101. PMID 6578235. S2CID 19490884.
- ↑ Pruitt KM, Tenovuo J, Andrews RW, McKane T (February 1982). "Lactoperoxidase-catalyzed oxidation of thiocyanate: polarographic study of the oxidation products". Biochemistry. 21 (3): 562–7. doi:10.1021/bi00532a023. PMID 7066307.
- ↑ Thomas EL, Pera KA, Smith KW, Chwang AK (February 1983). "Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system". Infect. Immun. 39 (2): 767–78. doi:10.1128/IAI.39.2.767-778.1983. PMC 348016 . PMID 6832819.
- ↑ Thomas EL (May 1981). "Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate". Biochemistry. 20 (11): 3273–80. doi:10.1021/bi00514a045. PMID 7248282.
- ↑ Thomas EL, Aune TM (May 1978). "Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action". Infect. Immun. 20 (2): 456–63. doi:10.1128/IAI.20.2.456-463.1978. PMC 421877 . PMID 352945.
- ↑ Tenovuo J (January 2002). "Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: efficacy and safety". Oral Dis. 8 (1): 23–9. doi:10.1034/j.1601-0825.2002.1o781.x. PMID 11936452.
- ↑ White WE, Pruitt KM, Mansson-Rahemtulla B (February 1983). "Peroxidase-Thiocyanate-Peroxide Antibacterial System Does Not Damage DNA". Antimicrob. Agents Chemother. 23 (2): 267–72. doi:10.1128/aac.23.2.267. PMC 186035 . PMID 6340603.
- ↑ Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, Conner GE (November 2009). "Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli". Free Radic. Biol. Med. 47 (10): 1450–8. doi:10.1016/j.freeradbiomed.2009.08.017. PMC 2767478 . PMID 19703552.
- ↑ "Public summary of positive opinion for orphan designation of hypothiocyanite / lactoferrin for the treatment of cystic fibrosis" (PDF). Pre-authorisation Evaluation of Medicines for Human Use. European Medicines Agency. 2009-09-07. Archived from the original (PDF) on 2010-05-30. Retrieved 2010-01-23.
- ↑ "Meveol: orphan drug status granted by the FDA for the treatment of cystic fibrosis". United States Food and Drug Administration. 2009-11-05. Archived from the original on 2009-12-24. Retrieved 2010-01-23.
- ↑ Mikola H, Waris M, Tenovuo J (Mar 1995). "Inhibition of herpes simplex virus type 1, respiratory syncytial virus and echovirus type 11 by peroxidase-generated hypothiocyanite". Antiviral Res. 26 (2): 161–71. doi:10.1016/0166-3542(94)00073-h. PMID 7605114.