A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are sometimes combined.

Smoking is the process of flavoring, browning, cooking, or preserving food by exposing it to smoke from burning or smoldering material, most often wood. Meat, fish, and lapsang souchong tea are often smoked.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Perfume is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of manmade mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory."

Salami is a cured sausage consisting of fermented and air-dried meat, typically pork. Historically, salami was popular among Southern, Eastern, and Central European peasants because it can be stored at room temperature for up to 45 days once cut, supplementing a potentially meager or inconsistent supply of fresh meat. Countries and regions across Europe make their own traditional varieties of salami.
Stearin, or tristearin, or glyceryl tristearate is an odourless, white powder. It is a triglyceride derived from three units of stearic acid. Most triglycerides are derived from at least two and more commonly three different fatty acids. Like other triglycerides, stearin can crystallise in three polymorphs. For stearin, these melt at 54 (α-form), 65, and 72.5 °C (β-form).

Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent FA in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C and a pKa of 4.50.

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D is 16:0. It is a major component of the oil from the fruit of oil palms, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats. Palmitates are the salts and esters of palmitic acid. The palmitate anion is the observed form of palmitic acid at physiologic pH (7.4). Major sources of C16:0 are palm oil, palm kernel oil, coconut oil, and milk fat.
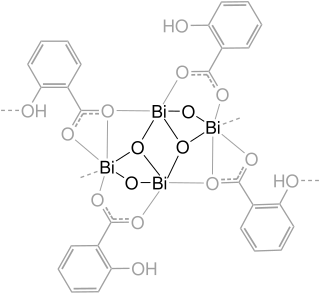
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indigestion, upset stomach, and diarrhea.
Ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.

Liquid smoke is a water-soluble yellow to red liquid used as a flavoring as a substitute for cooking with wood smoke while retaining a similar flavor. It can be used to flavor any meat or vegetable. It is available as pure condensed smoke from various types of wood, and as derivative formulas containing additives.

Citral is an acyclic monoterpene aldehyde. Being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the E-isomer is named geranial (trans-citral) or citral A. The Z-isomer is named neral (cis-citral) or citral B. These stereoisomers occur as a mixture, not necessarily racemic; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61.

Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the oil of citrus fruit peels. The D-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common L-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants.

Potassium sorbate is the potassium salt of sorbic acid, chemical formula CH3CH=CH−CH=CH−CO2K. It is a white salt that is very soluble in water (58.2% at 20 °C). It is primarily used as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal-care products. While sorbic acid occurs naturally in rowan and hippophae berries, virtually all of the world's supply of sorbic acid, from which potassium sorbate is derived, is manufactured synthetically.
Guaiacol is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs widely in nature and is a common product of the pyrolysis of wood.
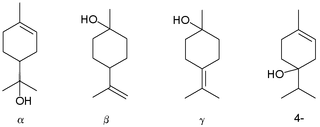
Terpineol is any of four isomeric monoterpenoids. Terpenoids are terpene that are modified by the addition of a functional group, in this case, an alcohol. Terpineols have been isolated from a variety of sources such as cardamom, cajuput oil, pine oil, and petitgrain oil. Four isomers exist: α-, β-, γ-terpineol, and terpinen-4-ol. β- and γ-terpineol differ only by the location of the double bond. Terpineol is usually a mixture of these isomers with α-terpineol as the major constituent.
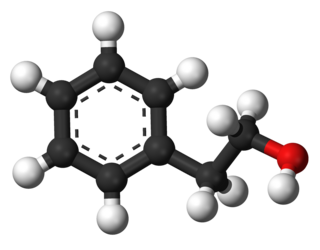
Phenethyl alcohol, or 2-phenylethanol, is an organic compound with the chemical formula C6H5CH2CH2OH. It is a colourless liquid with a pleasant floral odor. It occurs widely in nature, being found in a variety of essential oils. It is slightly soluble in water, but miscible with most organic solvents. The molecule of phenethyl alcohol consists of a phenethyl group attached to a hydroxyl group.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.

Linalyl acetate is an organic compound, the acetate ester of linalool and a phytochemical found in many flowers and spice plants. It is one of the principal components of the essential oils of bergamot and lavender. It often occurs together with linalool and is a widely used fragrance.
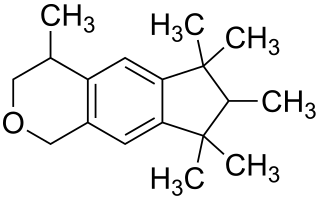
Galaxolide is a synthetic musk with a clean sweet musky floral woody odor used in fragrances. It is one of the musk components that perfume and cologne manufacturers use to add a musk odor to their products. Galaxolide was first synthesized in 1965, and used in the late 1960s in some fabric softeners and detergents. High concentrations were also incorporated in fine fragrances.