
In organic chemistry, a diene ; also diolefin, dy-OH-lə-fin) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alkene units, with the standard prefix di of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition.

The chemical industry comprises the companies and other organizations that develop and produce industrial, specialty and other chemicals. Central to the modern world economy, it converts raw materials into commodity chemicals for industrial and consumer products. It includes industries for petrochemicals such as polymers for plastics and synthetic fibers; inorganic chemicals such as acids and alkalis; agricultural chemicals such as fertilizers, pesticides and herbicides; and other categories such as industrial gases, speciality chemicals and pharmaceuticals.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.

Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene. These materials have good abrasion resistance and good aging stability when protected by additives. In 2012, more than 5.4 million tonnes of SBR were processed worldwide. About 50% of car tires are made from various types of SBR. The styrene/butadiene ratio influences the properties of the polymer: with high styrene content, the rubbers are harder and less rubbery. SBR is not to be confused with the thermoplastic elastomer, styrene-butadiene block copolymer, although being derived from the same monomers.

Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with designing and developing pharmaceutical drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR).

Polybutadiene [butadiene rubber, BR] is a synthetic rubber. It offers high elasticity, high resistance to wear, good strength even without fillers, and excellent abrasion resistance when filled and vulcanized. "Polybutadiene" is a collective name for homopolymers formed from the polymerization of the monomer 1,3-butadiene. The IUPAC refers to polybutadiene as "poly(buta-1,3-diene)". Historically, an early generation of synthetic polybutadiene rubber produced in Germany by Bayer using sodium as a catalyst was known as "Buna rubber". Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.
Elmer Keiser Bolton was an American chemist and research director for DuPont, notable for his role in developing neoprene and directing the research that led to the discovery of nylon.
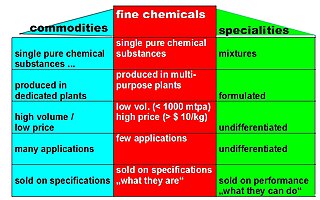
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg. The class of fine chemicals is subdivided either on the basis of the added value, or the type of business transaction, namely standard or exclusive products.
The Willard Gibbs Award, presented by the Chicago Section of the American Chemical Society, was established in 1910 by William A. Converse (1862–1940), a former Chairman and Secretary of the Chicago Section of the society and named for Professor Josiah Willard Gibbs (1839–1903) of Yale University. Gibbs, whose formulation of the Phase Rule founded a new science, is considered by many to be the only American-born scientist whose discoveries are as fundamental in nature as those of Newton and Galileo.
In 1957, the research organization of the Chemicals Department of E. I. du Pont de Nemours and Company was renamed Central Research Department, beginning the history of the premier scientific organization within DuPont and one of the foremost industrial laboratories devoted to basic science. Located primarily at the DuPont Experimental Station and Chestnut Run, in Wilmington, Delaware, it expanded to include laboratories in Geneva, Switzerland, Seoul, South Korea, Shanghai, China, and India (Hyderabad). In January, 2016 a major layoff marked the end of the organization.
Carl Shipp "Speed" Marvel was an American chemist who specialized in polymer chemistry. He made important contributions to U.S. synthetic rubber program during World War II, and later worked at developing polybenzimidazoles, temperature-resistant polymers that are used in the aerospace industry, in fire-fighting equipment, and as a replacement for asbestos. He has been described as "one of the world's outstanding organic chemists" and received numerous awards, including the 1956 Priestley Medal and the 1986 National Medal of Science, presented by President Ronald Reagan.

Moscow State University of Fine Chemical Technologies named after M.V. Lomonosov is one of the oldest universities in the country that offer training in a wide range of specialties in the field of chemical technology.

1,4-Dichlorobut-2-ene are organochlorine compounds with the formula ClCH2CH=CHCH2Cl. Cis and trans isomers exist. These compounds are intermediates in the industrial production of chloroprene. They are main impurity in technical grade chloroprene.
Commodity chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines and web sites such as Chemical Week and ICIS. There have been several studies of the scale and complexity of this market for example in the USA.
The A.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences was founded in 1954 by a prominent scientist, the President of the USSR Academy of Sciences, academician A. N. Nesmeyanov (1899–1980), who was a "father" of the modern chemistry of organoelement and organometallic compounds. He headed the Institute for 26 years. Major directions of research of the Institute are the following: Laboratories of Organoelement Profile, Laboratories of Polymer Profile, and Laboratories of Physical Profile.
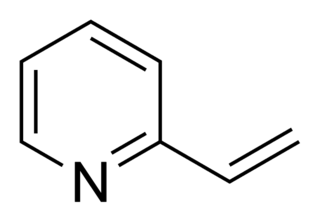
2-Vinylpyridine is an organic compound with the formula CH2CHC5H4N. It is a derivative of pyridine with a vinyl group in the 2-position, next to the nitrogen. It is a colorless liquid, although samples are often brown. It is used industrially as a precursor to specialty polymers and as an intermediate in the chemical, pharmaceutical, dye, and photo industries. Vinylpyridine is sensitive to polymerization. It may be stabilized with a polymerisation inhibitor such as tert-butylcatechol. Owing to its tendency to polymerize, samples are typically refrigerated.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.
Samuel Emmett Horne Jr. was a research scientist at B. F. Goodrich noted for first synthesizing cis-1,4-polyisoprene, the main polymer contained in natural tree rubber, using Ziegler catalysis. Earlier attempts to produce synthetic rubber from isoprene had been unsuccessful, but in 1955, Horne prepared 98 percent cis-1,4-polyisoprene via the stereospecific polymerization of isoprene. The product of this reaction differs from natural rubber only slightly. It contains a small amount of cis-1,2-polyisoprene, but it is indistinguishable from natural rubber in its physical properties.
Walter Bock was a German chemist who developed styrene-butadiene copolymer by emulsion polymerization as a synthetic rubber (SBR).













