
Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Amino acid biosynthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids.
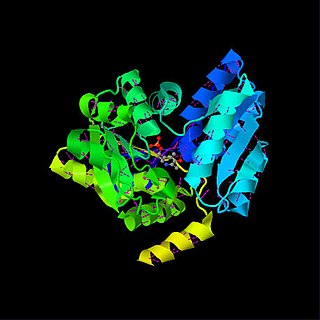
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structure and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes the deamination of L-serine to yield pyruvate, with the release of ammonia.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
In enzymology, a putrescine oxidase (EC 1.4.3.10) is an enzyme that catalyzes the chemical reaction
The enzyme 3-chloro-D-alanine dehydrochlorinase (EC 4.5.1.2) catalyzes the reaction
The enzyme carbamoyl-serine ammonia-lyase (EC 4.3.1.13) catalyzes the chemical reaction
The enzyme cysteine-S-conjugate β-lyase (EC 4.4.1.13) catalyzes the chemical reaction
The enzyme D-serine ammonia-lyase (EC 4.3.1.18), with systematic name D-serine ammonia-lyase (pyruvate-forming), catalyzes the chemical reaction
The enzyme Glucosaminate ammonia-lyase (EC 4.3.1.9) catalyzes the chemical reaction
The enzyme homocysteine desulfhydrase (EC 4.4.1.2) catalyzes the chemical reaction

The enzyme methionine γ-lyase (EC 4.4.1.11, MGL) is in the γ-family of PLP-dependent enzymes. It degrades sulfur-containing amino acids to α-keto acids, ammonia, and thiols:

Threonine ammonia-lyase (EC 4.3.1.19, systematic name L-threonine ammonia-lyase (2-oxobutanoate-forming), also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into α-ketobutyrate and ammonia:

The enzyme anthranilate synthase catalyzes the chemical reaction
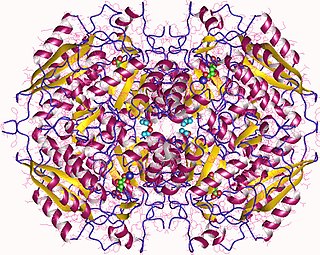
The enzyme glucarate dehydratase (EC 4.2.1.40) catalyzes the chemical reaction
In enzymology, a formimidoylglutamate deiminase (EC 3.5.3.13) is an enzyme that catalyzes the chemical reaction
In enzymology, an O-acetylhomoserine aminocarboxypropyltransferase is an enzyme that catalyzes the chemical reaction
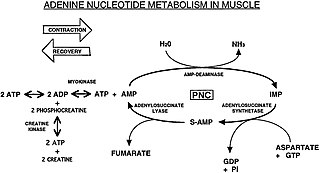
The Purine Nucleotide Cycle is a metabolic pathway in protein metabolism requiring the amino acids aspartate and glutamate. The cycle is used to regulate the levels of adenine nucleotides, in which ammonia and fumarate are generated. AMP converts into IMP and the byproduct ammonia. IMP converts to S-AMP (adenylosuccinate), which then converts to AMP and the byproduct fumarate. The fumarate goes on to produce ATP (energy) via oxidative phosphorylation as it enters the Krebs cycle and then the electron transport chain. Lowenstein first described this pathway and outlined its importance in processes including amino acid catabolism and regulation of flux through glycolysis and the Krebs cycle.
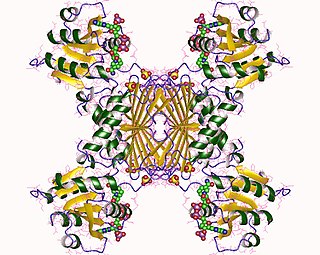
In enzymology, a 4-hydroxy-tetrahydrodipicolinate reductase (EC 1.17.1.8) is an enzyme that catalyzes the chemical reaction











