
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in our diet. In plants isoleucine can be synthesized from threonine and methionine. In plants and bacteria, isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes. It is encoded by the codons AUU, AUC, and AUA.
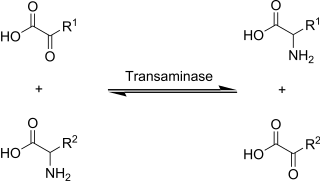
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids.

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.
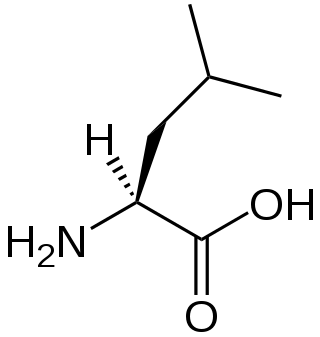
A branched-chain amino acid (BCAA) is an amino acid having an aliphatic side-chain with a branch. Among the proteinogenic amino acids, there are three BCAAs: leucine, isoleucine, and valine. Non-proteinogenic BCAAs include 2-aminoisobutyric acid and alloisoleucine.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
Branched-chain amino acid aminotransferase (BCAT), also known as branched-chain amino acid transaminase, is an aminotransferase enzyme (EC 2.6.1.42) which acts upon branched-chain amino acids (BCAAs). It is encoded by the BCAT2 gene in humans. The BCAT enzyme catalyzes the conversion of BCAAs and α-ketoglutarate into branched chain α-keto acids and glutamate.
In enzymology, a ketol-acid reductoisomerase (EC 1.1.1.86) is an enzyme that catalyzes the chemical reaction

In enzymology, an (S)-2-hydroxy-acid oxidase (EC 1.1.3.15) is an enzyme that catalyzes the chemical reaction

In enzymology, a leucine dehydrogenase (EC 1.4.1.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2,5-diaminovalerate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-hydroxyglutamate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a 5-aminovalerate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a cysteine-conjugate transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a D-amino-acid transaminase is an enzyme that catalyzes the chemical reaction:
In enzymology, glutamate-prephenate aminotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a glycine transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a (S)-3-amino-2-methylpropionate transaminase is an enzyme that catalyzes the chemical reaction

In enzymology, a tryptophan transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a valine-3-methyl-2-oxovalerate transaminase is an enzyme that catalyzes the chemical reaction

Branched chain amino acid transaminase 1 is a protein that in humans is encoded by the BCAT1 gene. It is the first enzyme in the branched-chain amino acid (BCAA) degradation pathway and facilitates the reversible transamination of BCAAs and glutamate. BCAT1 resides in the cytoplasm, while its isoform, BCAT2, is found in the mitochondria.











