
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.
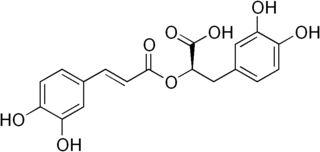
Rosmarinic acid, named after rosemary, is a polyphenol constituent of many culinary herbs, including rosemary, perilla, sage, mint, and basil.
Enoyl-acyl carrier protein reductase, is a key enzyme of the type II fatty acid synthesis (FAS) system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species. In addition, the bacterial ENR sequence and structural organization are distinctly different from those of mammalian fatty acid biosynthesis enzymes.
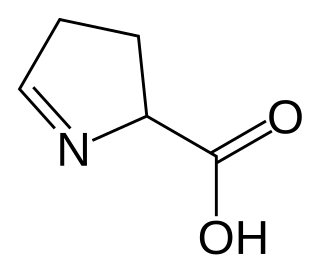
1-Pyrroline-5-carboxylic acid is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA).
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction
In enzymology, a retinol dehydrogenase (RDH) (EC 1.1.1.105) is an enzyme that catalyzes the chemical reaction
In enzymology, a leucocyanidin oxygenase (EC 1.14.11.19) is an enzyme that catalyzes the chemical reaction
In enzymology, a leucoanthocyanidin reductase (EC 1.17.1.3) (LAR, aka leucocyanidin reductase or LCR) is an enzyme that catalyzes the chemical reaction
In enzymology, a stizolobate synthase (EC 1.13.11.29) is an enzyme that catalyzes the chemical reaction
In enzymology, a stizolobinate synthase (EC 1.13.11.30) is an enzyme that catalyzes the chemical reaction

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.

Aromadendrin is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.
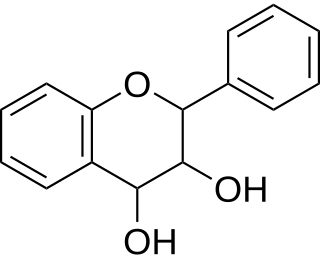
Leucoanthocyanidin (flavan-3,4-diols) are colorless chemical compounds related to anthocyanidins and anthocyanins. Leucoanthocyanins can be found in Anadenanthera peregrina and in several species of Nepenthes including N. burbidgeae, N. muluensis, N. rajah, N. tentaculata, and N. × alisaputrana.

Leucocyanidin is a colorless chemical compound that is a member of the class of natural products known as leucoanthocyanidins.
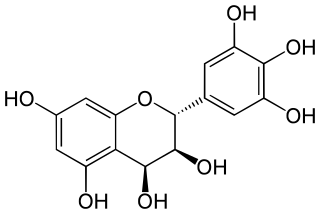
Leucodelphinidin is a colorless chemical compound related to leucoanthocyanidins. It can be found in Acacia auriculiformis, in the bark of Karada and in the kino (gum) from Eucalyptus pilularis.
The biosynthesis of phenylpropanoids involves a number of enzymes.
Phytoene desaturase (3,4-didehydrolycopene-forming) is an enzyme with systematic name 15-cis-phytoene:acceptor oxidoreductase (3,4-didehydrolycopene-forming). This enzyme catalyses the following chemical reaction











