
Aniracetam, also known as N-anisoyl-2-pyrrolidinone, is a racetam which is sold in Europe as a prescription drug. It is not approved by the Food and Drug Administration for use in the United States as a prescription medication or dietary supplement. Despite the FDA's lack of approval, the drug is readily available over-the-counter in misbranded dietary supplements.

Benzoctamine is a drug that possesses sedative and anxiolytic properties. Marketed as Tacitin by Ciba-Geigy, it is different from most sedative drugs because in most clinical trials it does not produce respiratory depression, but actually stimulates the respiratory system. As a result, when compared to other sedative and anxiolytic drugs such as benzodiazepines like diazepam, it is a safer form of tranquilizing. However, when co-administered with other drugs that cause respiratory depression, like morphine, it can cause increased respiratory depression.

Abecarnil (ZK-112,119) is an anxiolytic drug from the β-Carboline family. It is one of a relatively recently developed class of medicines known as the nonbenzodiazepines, which have similar effects to the older benzodiazepine group, but with quite different chemical structures. It is a partial agonist acting selectively at the benzodiazepine site of the GABAA receptor.

SX-3228 is a sedative and hypnotic drug used in scientific research. It has similar effects to sedative-hypnotic benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine hypnotic.

The elevated plus maze (EPM) is a test measuring anxiety in laboratory animals that usually uses rodents as a screening test for putative anxiolytic or anxiogenic compounds and as a general research tool in neurobiological anxiety research such as PTSD and TBI. The model is based on the test animal's aversion to open spaces and tendency to be thigmotaxic. In the EPM, this anxiety is expressed by the animal spending more time in the enclosed arms. The validity of the model has been criticized as non-classical clinical anxiolytics produce mixed results in the EPM test. Despite this, the model is still commonly used for screening putative anxiolytics and for general research into the brain mechanisms of anxiety.
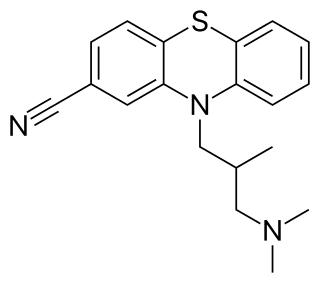
Cyamemazine (Tercian), also known as cyamepromazine, is a typical antipsychotic drug of the phenothiazine class which was introduced by Theraplix in France in 1972 and later in Portugal as well.

Zacopride is a potent antagonist at the 5-HT3 receptor and an agonist at the 5-HT4 receptor. It has anxiolytic and nootropic effects in animal models, with the (R)-(+)-enantiomer being the more active form. It also has antiemetic and pro-respiratory effects, both reducing sleep apnea and reversing opioid-induced respiratory depression in animal studies. Early animal trials have also revealed that administration of zacopride can reduce preference for and consumption of ethanol.
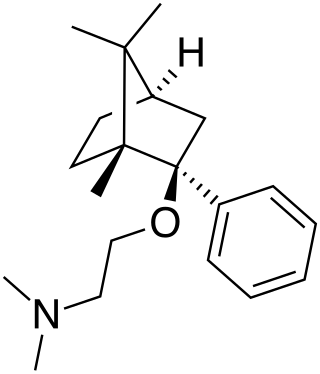
Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.

GR-159897 is a potent and selective NK2 receptor antagonist drug. It has anxiolytic effects in animal models, and also inhibits bronchoconstriction of the airways, which may potentially make it useful in the treatment of asthma.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

Conditioned place preference (CPP) is a form of Pavlovian conditioning used to measure the motivational effects of objects or experiences. This motivation comes from the pleasurable aspect of the experience, so that the brain can be reminded of the context that surrounded the "encounter". By measuring the amount of time an animal spends in an area that has been associated with a stimulus, researchers can infer the animal's liking for the stimulus. This paradigm can also be used to measure conditioned place aversion with an identical procedure involving aversive stimuli instead. Both procedures usually involve mice or rats as subjects. This procedure can be used to measure extinction and reinstatement of the conditioned stimulus. Certain drugs are used in this paradigm to measure their reinforcing properties. Two different methods are used to choose the compartments to be conditioned, and these are biased vs. unbiased. The biased method allows the animal to explore the apparatus, and the compartment they least prefer is the one that the drug is administered in and the one they most prefer is the one where the vehicle is injected. This method allows the animal to choose the compartment they get the drug and vehicle. In comparison, the unbiased method does not allow the animal to choose what compartment they get the drug and vehicle in. Instead, the researcher chooses the compartments.

SB-216641 is a drug which is a selective antagonist for the serotonin receptor 5-HT1B, with around 25x selectivity over the closely related 5-HT1D receptor. It is used in scientific research, and has demonstrated anxiolytic effects in animal studies.

The conflict procedure is an experiment often used in scientific research to quantify anxiety levels by measuring changes in punished/unpunished responses. It is often used to screen drugs for their potential to inhibit anxiety.

Osemozotan (MKC-242) is a selective 5-HT1A receptor agonist with some functional selectivity, acting as a full agonist at presynaptic and a partial agonist at postsynaptic 5-HT1A receptors. 5-HT1A receptor stimulation influences the release of various neurotransmitters including serotonin, dopamine, norepinephrine, and acetylcholine. 5-HT1A receptors are inhibitory G protein-coupled receptor.
The hot plate test is a test of the pain response in animals, similar to the tail flick test. Both hot plate and tail-flick methods are used generally for centrally acting analgesic, while peripherally acting drugs are ineffective in these tests but sensitive to acetic acid-induced writhing test.

Animal models of depression are research tools used to investigate depression and action of antidepressants. They are used as a simulation to investigate the symptomatology and pathophysiology of depressive illness and to screen novel antidepressants. These models provide insights into molecular, genetic, and epigenetic factors associated with depression. Criteria for valid animal models include face, construct, and predictive validity. Endophenotypes, such as anhedonia, behavioral despair, changes in appetite, neuroanatomical alterations, neuroendocrine disturbances, alterations in sleep architecture, and anxiety-related behaviors, are evaluated in these models. Antidepressant screening tests are employed to assess the effects of genetic, pharmacological, or environmental manipulations. Stress models including learned helplessness, chronic mild stress, and social defeat stress simulate the impact of stressors on depression. Early life stress models, psychostimulant withdrawal models, olfactory bulbectomy, and genetically engineered mice contribute to a comprehensive understanding of depression's etiology and potential therapeutic interventions.
Developed by Calvin S. Hall, the open field test is an experimental test used to assay general locomotor activity levels, anxiety, and willingness to explore in animals in scientific research. However, the extent to which behavior in the open field measures anxiety is controversial. The open field test can be used to assess memory by evaluating the ability of the animal to recognize a stimulus or object. Another animal test that is used to assess memory using that same concept is the novel object recognition test.
The hole-board test (HBT) is an experimental method used in scientific research to measure anxiety, stress, neophilia and emotionality in animals. Because of its ability to measure multiple behaviors it is a popular test in behavioral pharmacology, but the results are controversial.

Marble burying is an animal model used in scientific research to depict anxiety or obsessive–compulsive disorder (OCD) behavior. It is based on the observation that rats and mice will bury either harmful or harmless objects in their bedding. While widely used there is significant controversy over the interpretation of its results.
The Vogel conflict test (VCT) is a conflict based experimental method primarily used in pharmacology. It is used to determine anxiolytic properties of drugs. The VCT predicts drugs that can manage generalized anxiety disorders and acute anxiety states.














