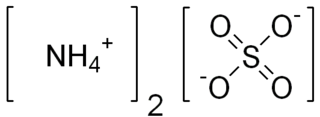Soil pH is a measure of the acidity or basicity (alkalinity) of a soil. Soil pH is a key characteristic that can be used to make informative analysis both qualitative and quantitatively regarding soil characteristics. pH is defined as the negative logarithm (base 10) of the activity of hydronium ions in a solution. In soils, it is measured in a slurry of soil mixed with water, and normally falls between 3 and 10, with 7 being neutral. Acid soils have a pH below 7 and alkaline soils have a pH above 7. Ultra-acidic soils and very strongly alkaline soils are rare.

Physiological plant disorders are caused by non-pathological conditions such as poor light, adverse weather, water-logging, phytotoxic compounds or a lack of nutrients, and affect the functioning of the plant system. Physiological disorders are distinguished from plant diseases caused by pathogens, such as a virus or fungus. While the symptoms of physiological disorders may appear disease-like, they can usually be prevented by altering environmental conditions. However, once a plant shows symptoms of a physiological disorder, it is likely that that season's growth or yield will be reduced.
Boron deficiency is a common deficiency of the micronutrient boron in plants. It is the most widespread micronutrient deficiency around the world and causes large losses in crop production and crop quality. Boron deficiency affects vegetative and reproductive growth of plants, resulting in inhibition of cell expansion, death of meristem, and reduced fertility.

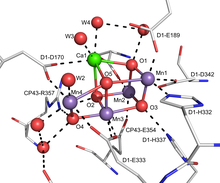
Calcium (Ca) deficiency is a plant disorder that can be caused by insufficient level of biologically available calcium in the growing medium, but is more frequently a product of low transpiration of the whole plant or more commonly the affected tissue. Plants are susceptible to such localized calcium deficiencies in low or non-transpiring tissues because calcium is not transported in the phloem. This may be due to water shortages, which slow the transportation of calcium to the plant, poor uptake of calcium through the stem, or too much nitrogen in the soil.

Iron (Fe) deficiency is a plant disorder also known as "lime-induced chlorosis". It can be confused with manganese deficiency. Soil iron concentration is high, but can become unavailable for absorption if soil pH is higher than 6.5. Excess of elements such as manganese in the soil can interfere with plant iron uptake triggering iron deficiency.

Nitrogen deficiency is a deficiency of nitrogen in plants. This can occur when organic matter with high carbon content, such as sawdust, is added to soil. Soil organisms use any nitrogen available to break down carbon sources, making nitrogen unavailable to plants. This is known as "robbing" the soil of nitrogen. All vegetables apart from nitrogen fixing legumes are prone to this disorder.

Potassium deficiency, also known as potash deficiency, is a plant disorder that is most common on light, sandy soils, because potassium ions (K+) are highly soluble and will easily leach from soils without colloids. Potassium deficiency is also common in chalky or peaty soils with a low clay content. It is also found on heavy clays with a poor structure.

Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Soil test may refer to one or more of a wide variety of soil analysis conducted for one of several possible reasons. Possibly the most widely conducted soil tests are those done to estimate the plant-available concentrations of plant nutrients, in order to determine fertilizer recommendations in agriculture. Other soil tests may be done for engineering (geotechnical), geochemical or ecological investigations.

Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig’s law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil.

Citrus production encompasses the production of citrus fruit, which are the highest-value fruit crop in terms of international trade. There are two main markets for citrus fruit:

In botany, chlorosis is a condition in which leaves produce insufficient chlorophyll. As chlorophyll is responsible for the green color of leaves, chlorotic leaves are pale, yellow, or yellow-white. The affected plant has little or no ability to manufacture carbohydrates through photosynthesis and may die unless the cause of its chlorophyll insufficiency is treated and this may lead to a plant diseases called rusts, although some chlorotic plants, such as the albino Arabidopsis thaliana mutant ppi2, are viable if supplied with exogenous sucrose.
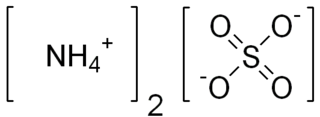
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a compound such as aluminium sulfate, which reacts in the soil to release protons. Acidification also occurs when base cations such as calcium, magnesium, potassium and sodium are leached from the soil.
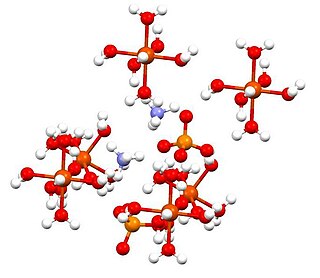
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2(H2O)6. Containing two different cations, Fe2+ and NH+4, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite.

Sugarcane grassy shoot disease (SCGS), is associated with 'Candidatus Phytoplasma sacchari' which are small, pleomorphic, pathogenic mycoplasma that contributes to yield losses from 5% up to 20% in sugarcane. These losses are higher in the ratoon crop. A higher incidence of SCGS has been recorded in some parts of Southeast Asia and India, resulting in 100% loss in cane yield and sugar production.
Necrotic ring spot is a common disease of turf caused by soil borne fungi that mainly infects roots (4). It is an important disease as it destroys the appearance of turfgrasses on park, playing fields and golf courses. Necrotic Ring Spot is caused by a fungal pathogen that is an ascomycete that produces ascospores in an ascocarp (6). They survive over winter, or any unfavorable condition as sclerotia. Most infection occurs in spring and fall when the temperature is about 13 to 28°C (5). The primary hosts of this disease are cool-season grasses such as Kentucky bluegrass and annual bluegrass (6). Once turf is infected with O. korrae, it kills turf roots and crowns. Symptoms of the disease are quite noticeable since they appear as large yellow ring-shaped patches of dead turf. Management of the disease is often uneasy and requires application of multiple controls. The disease can be controlled by many different kind of controls including chemicals and cultural.

Zinc deficiency occurs when plant growth is limited because the plant cannot take up sufficient quantities of this essential micronutrient from its growing medium. Zinc is one of the most important micronutrients.

Molybdenum (Mo) deficiency occurs when plant growth is limited because the plant cannot take up sufficient quantities of this essential micronutrient from its growing medium. For crops growing in soil, this may be a result of low concentrations of Mo in the soil as a whole, or because the soil Mo is held in forms that are not available to plants – sorption of Mo is strongest in acid soils.
Seventeen elements or nutrients are essential for plant growth and reproduction. They are carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), iron (Fe), boron (B), manganese (Mn), copper (Cu), zinc (Zn), molybdenum (Mo), nickel (Ni) and chlorine (Cl). Nutrients required for plants to complete their life cycle are considered essential nutrients. Nutrients that enhance the growth of plants but are not necessary to complete the plant's life cycle are considered non-essential. With the exception of carbon, hydrogen and oxygen, which are supplied by carbon dioxide and water, and nitrogen, provided through nitrogen fixation, the nutrients derive originally from the mineral component of the soil. The Law of the Minimum expresses that when the available form of a nutrient is not in enough proportion in the soil solution, then other nutrients cannot be taken up at an optimum rate by a plant. A particular nutrient ratio of the soil solution is thus mandatory for optimizing plant growth, a value which might differ from nutrient ratios calculated from plant composition.