Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.
The urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Glutamine is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral, polar amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases, and glutamine must be obtained from the diet. It is encoded by the codons CAA and CAG. It is named after glutamic acid, which in turn is named after its discovery in cereal proteins, gluten.

Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula NH+4 or [NH4]+. It is formed by the addition of a proton to ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups. Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it.

Iron (Fe) deficiency is a plant disorder also known as "lime-induced chlorosis". It can be confused with manganese deficiency. If soil iron concentration is high, in spite of this it can become unavailable for absorption if soil pH is higher than 6.5. Excess of elements such as manganese in the soil can interfere with plant iron uptake triggering iron deficiency.

Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig's law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil.
Nutrient sensing is a cell's ability to recognize and respond to fuel substrates such as glucose. Each type of fuel used by the cell requires an alternate pathway of utilization and accessory molecules such as enzymes and cofactors. In order to conserve resources a cell will only produce molecules that it needs at the time. The level and type of fuel that is available to a cell will determine the type of enzymes it needs to express from its genome for utilization. Receptors on the cell membrane's surface designed to be activated in the presence of specific fuel molecules communicate to the cell nucleus via a means of cascading interactions. Nutrient receptors are receptors that are primarily designed to perform the function of nutrient sensing, whereas other receptors are extensively multifunctional and perform many functions besides nutrient sensing. In this way the cell is aware of the available nutrients and is able to produce only the molecules specific to that nutrient type.

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.

Glutamine synthetase (GS) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:

Nitrate reductases are molybdoenzymes that reduce nitrate to nitrite. This reaction is critical for the production of protein in most crop plants, as nitrate is the predominant source of nitrogen in fertilized soils.
Natronomonas is a genus of the Halobacteriaceae.
In enzymology, a glutamate synthase (NADH) (EC 1.4.1.14) is an enzyme that catalyzes the chemical reaction
Glutaminolysis (glutamine + -lysis) is a series of biochemical reactions by which the amino acid glutamine is lysed to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate.
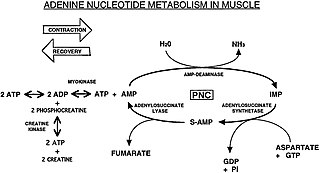
The Purine Nucleotide Cycle is a metabolic pathway in protein metabolism requiring the amino acids aspartate and glutamate. The cycle is used to regulate the levels of adenine nucleotides, in which ammonia and fumarate are generated. AMP converts into IMP and the byproduct ammonia. IMP converts to S-AMP (adenylosuccinate), which then converts to AMP and the byproduct fumarate. The fumarate goes on to produce ATP (energy) via oxidative phosphorylation as it enters the Krebs cycle and then the electron transport chain. Lowenstein first described this pathway and outlined its importance in processes including amino acid catabolism and regulation of flux through glycolysis and the Krebs cycle.
In biological oceanography, new production is supported by nutrient inputs from outside the euphotic zone, especially upwelling of nutrients from deep water, but also from terrestrial and atmosphere sources. New production depends on mixing and vertical advective processes associated with the circulation.

Yeast assimilable nitrogen or YAN is the combination of free amino nitrogen (FAN), ammonia (NH3) and ammonium (NH4+) that is available for a yeast, e.g. the wine yeast Saccharomyces cerevisiae, to use during fermentation. Outside of the fermentable sugars glucose and fructose, nitrogen is the most important nutrient needed to carry out a successful fermentation that doesn't end prior to the intended point of dryness or sees the development of off-odors and related wine faults. To this extent winemakers will often supplement the available YAN resources with nitrogen additives such as diammonium phosphate (DAP).
Glutamate synthase is an enzyme and frequently abbreviated as GOGAT. This enzyme manufactures glutamate from glutamine and α-ketoglutarate, and thus along with glutamine synthetase plays a central role in the regulation of nitrogen assimilation in photosynthetic eukaryotes and prokaryotes. This is of great importance as primary productivity in many marine environments is regulated by the availability of inorganic nitrogen.
Seventeen elements or nutrients are essential for plant growth and reproduction. They are carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P), potassium (K), sulfur (S), calcium (Ca), magnesium (Mg), iron (Fe), boron (B), manganese (Mn), copper (Cu), zinc (Zn), molybdenum (Mo), nickel (Ni) and chlorine (Cl). Nutrients required for plants to complete their life cycle are considered essential nutrients. Nutrients that enhance the growth of plants but are not necessary to complete the plant's life cycle are considered non-essential, although some of them, such as silicon (Si), have been shown to improve nutrent availability, hence the use of stinging nettle and horsetail macerations in Biodynamic agriculture. With the exception of carbon, hydrogen and oxygen, which are supplied by carbon dioxide and water, and nitrogen, provided through nitrogen fixation, the nutrients derive originally from the mineral component of the soil. The Law of the Minimum expresses that when the available form of a nutrient is not in enough proportion in the soil solution, then other nutrients cannot be taken up at an optimum rate by a plant. A particular nutrient ratio of the soil solution is thus mandatory for optimizing plant growth, a value which might differ from nutrient ratios calculated from plant composition.











