
α-Ketoglutaric acid is one of two ketone derivatives of glutaric acid. The term "ketoglutaric acid," when not further qualified, almost always refers to the alpha variant. β-Ketoglutaric acid varies only by the position of the ketone functional group, and is much less common.
Nitrogen assimilation is the formation of organic nitrogen compounds like amino acids from inorganic nitrogen compounds present in the environment. Organisms like plants, fungi and certain bacteria that can fix nitrogen gas (N2) depend on the ability to assimilate nitrate or ammonia for their needs. Other organisms, like animals, depend entirely on organic nitrogen from their food.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
Ferredoxins are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.

In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.
15,16-dihydrobiliverdin:ferredoxin oxidoreductase is an enzyme that catalyzes the following chemical reaction
In enzymology, a phycocyanobilin:ferredoxin oxidoreductase is an enzyme that catalyzes the chemical reaction
In enzymology, a phycoerythrobilin:ferredoxin oxidoreductase is an enzyme that catalyzes the chemical reaction
In enzymology, a phytochromobilin:ferredoxin oxidoreductase is an enzyme that catalyzes the chemical reaction
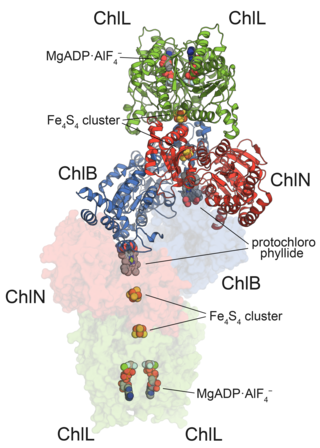
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide a. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
In enzymology, a 2-oxoglutarate synthase (EC 1.2.7.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-methyl-2-oxobutanoate dehydrogenase (ferredoxin) (EC 1.2.7.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a carbon-monoxide dehydrogenase (ferredoxin) (EC 1.2.7.4) is an enzyme that catalyzes the chemical reaction

In enzymology, a pyruvate synthase is an enzyme that catalyzes the interconversion of pyruvate and acetyl-CoA. It is also called pyruvate:ferredoxin oxidoreductase (PFOR).
In enzymology, a ferredoxin-NADP+ reductase (EC 1.18.1.2) abbreviated FNR, is an enzyme that catalyzes the chemical reaction
In enzymology, a ferredoxin—nitrate reductase (EC 1.7.7.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a ferredoxin—nitrite reductase (EC 1.7.7.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a glutamate synthase (NADH) (EC 1.4.1.14) is an enzyme that catalyzes the chemical reaction

In enzymology, a glutamate synthase (NADPH) (EC 1.4.1.13) is an enzyme that catalyzes the chemical reaction
Glutamate synthase is an enzyme and frequently abbreviated as GOGAT. This enzyme manufactures glutamate from glutamine and α-ketoglutarate, and thus along with glutamine synthetase plays a central role in the regulation of nitrogen assimilation in photosynthetic eukaryotes and prokaryotes. This is of great importance as primary productivity in many marine environments is regulated by the availability of inorganic nitrogen.





