
Mesquite is a common name for several plants in the genus Prosopis, which contains over 40 species of small leguminous trees. They are native to dry areas in the Americas. They have extremely long roots to seek water from very far under ground. As a legume, mesquites are one of the few sources of fixed nitrogen in the desert habitat. These trees bloom from spring to summer. They often produce fruits known as "pods". Prosopis spp. are able to grow up to 8 metres (26 ft) tall, depending on site and climate. They are deciduous and depending on location and rainfall have either deep or shallow roots. Prosopis is considered long-lived because of the low mortality rate after the dicotyledonous stage and juveniles are also able to survive in conditions with low light and drought. The Cahuilla indigenous people of western North America were known to eat the seeds of mesquite.

Prosopis is a genus of flowering plants in the family Fabaceae. It contains around 45 species of spiny trees and shrubs found in subtropical and tropical regions of the Americas, Africa, Western Asia, and South Asia. They often thrive in arid soil and are resistant to drought, on occasion developing extremely deep root systems. Their wood is usually hard, dense and durable. Their fruits are pods and may contain large amounts of sugar. The generic name means "burdock" in late Latin and originated in the Greek language.

Prosopis juliflora is a shrub or small tree in the family Fabaceae, a kind of mesquite. It is native to Mexico, South America and the Caribbean. It has become established as an invasive weed in Africa, Asia, Australia and elsewhere. It is a contributing factor to continuing transmission of malaria, especially during dry periods when sugar sources from native plants are largely unavailable to mosquitoes.
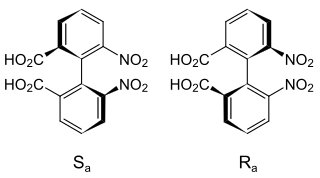
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers. They occur naturally and are important in pharmaceutical design. When the substituents are achiral, these conformers are enantiomers (atropoenantiomers), showing axial chirality; otherwise they are diastereomers (atropodiastereomers).
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.

Prosopis glandulosa, commonly known as honey mesquite, is a species of small to medium-sized, thorny shrub or tree in the legume family (Fabaceae).

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

Cistus salviifolius, common names sage-leaved rock-rose, salvia cistus or Gallipoli rose, is a shrub of the family Cistaceae.

Leucocyanidin is a colorless chemical compound that is a member of the class of natural products known as leucoanthocyanidins.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.
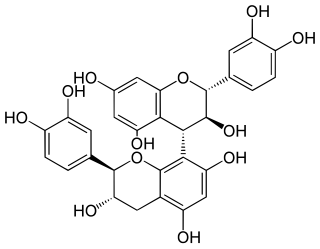
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer.
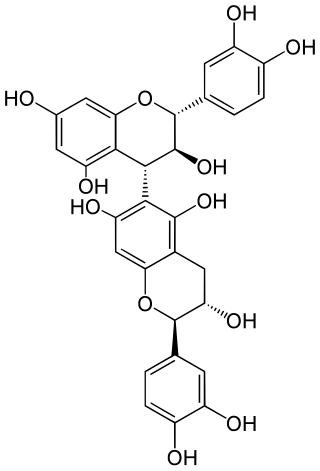
Procyanidin B6 is a B type proanthocyanidin.

Cassia abbreviata, commonly known as the sjambok pod or long-tail cassia, is a mostly tropical tree species in the genus Cassia, which is native to Africa.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.
Decarboxylative cross coupling reactions are chemical reactions in which a carboxylic acid is reacted with an organic halide to form a new carbon-carbon bond, concomitant with loss of CO2. Aryl and alkyl halides participate. Metal catalyst, base, and oxidant are required.

Oritin is a flavan-3-ol, a type of flavonoid. It is a component of the proteracacinidin tannins of Acacia galpinii and Acacia caffra.
β-Carbon elimination is a type of reaction in organometallic chemistry wherein an allyl ligand bonded to a metal center is broken into the corresponding metal-bonded alkyl (aryl) ligand and an alkene. It is a subgroup of elimination reactions. Though less common and less understood than β-hydride elimination, it is an important step involved in some olefin polymerization processes and transition-metal-catalyzed organic reactions.















