The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in the presence of a base and a palladium catalyst to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes.
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of noble metal catalysis in organic synthesis. This reaction is sometimes telescoped with the related Miyaura borylation; the combination is the Suzuki–Miyaura reaction. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls.
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) HC≡CH by metallic or other cations. The term is also used, more loosely, for any compound obtained in the same way from an acetylene derivative RC≡CH, where R is some organic side chain.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.
The Ullmann reaction or Ullmann coupling, named after Fritz Ullmann, couples two aryl or alkyl groups with the help of copper. The reaction was first reported by Ullmann and his student Bielecki in 1901. It has been later shown that palladium and nickel can also be effectively used.

Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry.

Richard Frederick Heck was an American chemist noted for the discovery and development of the Heck reaction, which uses palladium to catalyze organic chemical reactions that couple aryl halides with alkenes. The analgesic naproxen is an example of a compound that is prepared industrially using the Heck reaction.
Unlike its lighter congeners, the halogen iodine forms a number of stable organic compounds, in which iodine exhibits higher formal oxidation states than -1 or coordination number exceeding 1. These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.

Trimethylsilylacetylene is the organosilicon compound with the formula (CH3)3SiC2H. A colorless liquid, "tms acetylene", as it is also called, is used as a source of "HC2−" in organic synthesis.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.

In organometallic chemistry, palladium-NHC complexes are a family of organopalladium compounds in which palladium forms a coordination complex with N-heterocyclic carbenes (NHCs). They have been investigated for applications in homogeneous catalysis, particularly cross-coupling reactions.

Methyl 2-bromoacetate (methyl bromoactate) is a chemical compound with the molecular formula C3H5BrO2.

Togni reagent II is a chemical compound used in organic synthesis for direct electrophilic trifluoromethylation.
Miyaura borylation, also known as the Miyaura borylation reaction, is a named reaction in organic chemistry that allows for the generation of boronates from vinyl or aryl halides with the cross-coupling of bis(pinacolato)diboron in basic conditions with a catalyst such as PdCl2(dppf). The resulting borylated products can be used as coupling partners for the Suzuki reaction.
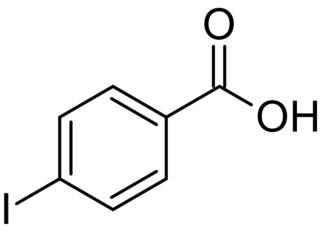
4-Iodobenzoic acid, or p-iodobenzoic acid, is an isomer of iodobenzoic acid.

4-Bromobenzaldehyde, or p-bromobenzaldehyde, is an organobromine compound with the formula BrC6H4CHO. It is one of three isomers of bromobenzaldehyde. 4-Bromobenzaldehyde is a colorless liquid. It displays reactivity characteristic of benzaldehyde and an aryl bromide.

4-Ethynylbenzaldehyde, or p-ethynylbenzaldehyde, is an organic compound with the formula HC2C6H4COH. It is an ethynyl derivative of benzaldehyde, or may also be viewed as a formylated derivative of phenylacetylene.

1-Bromo-4-iodobenzene is a mixed aryl halide (aryl bromide and aryl iodide) with the formula BrC6H4I.
















