Related Research Articles
General anaesthetics are often defined as compounds that induce a loss of consciousness in humans or loss of righting reflex in animals. Clinical definitions are also extended to include an induced coma that causes lack of awareness to painful stimuli, sufficient to facilitate surgical applications in clinical and veterinary practice. General anaesthetics do not act as analgesics and should also not be confused with sedatives. General anaesthetics are a structurally diverse group of compounds whose mechanisms encompass multiple biological targets involved in the control of neuronal pathways. The precise workings are the subject of some debate and ongoing research.

A general anaesthetic is a drug that brings about a reversible loss of consciousness. These drugs are generally administered by an anaesthetist/anesthesiologist to induce or maintain general anaesthesia to facilitate surgery.
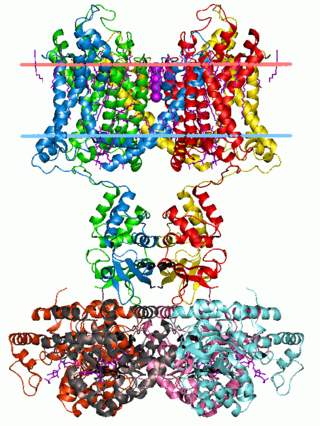
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.

The two-pore-domain or tandem pore domain potassium channels are a family of 15 members that form what is known as leak channels which possess Goldman-Hodgkin-Katz (open) rectification. These channels are regulated by several mechanisms including signaling lipids, oxygen tension, pH, mechanical stretch, and G-proteins. Two-pore-domain potassium channels correspond structurally to a inward-rectifier potassium channel α-subunits. Each inward-rectifier potassium channel α-subunit is composed of two transmembrane α-helices, a pore helix and a potassium ion selectivity filter sequence and assembles into a tetramer forming the complete channel. The two-pore domain potassium channels instead are dimers where each subunit is essentially two α-subunits joined together.

Kv7.2 (KvLQT2) is a voltage- and lipid-gated potassium channel protein coded for by the gene KCNQ2.

Acid-sensing ion channel 1 (ASIC1) also known as amiloride-sensitive cation channel 2, neuronal (ACCN2) or brain sodium channel 2 (BNaC2) is a protein that in humans is encoded by the ASIC1 gene. The ASIC1 gene is one of the five paralogous genes that encode proteins that form trimeric acid-sensing ion channels (ASICs) in mammals. The cDNA of this gene was first cloned in 1996. The ASIC genes have splicing variants that encode different proteins that are called isoforms.

Potassium channel subfamily K member 2, also known as TREK-1, is a protein that in humans is encoded by the KCNK2 gene.
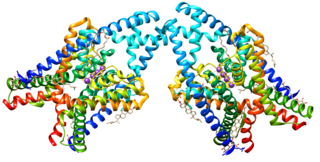
Potassium channel subfamily K member 3 is a protein that in humans is encoded by the KCNK3 gene.

Potassium channel subfamily K member 4 is a protein that in humans is encoded by the KCNK4 gene. KCNK4 protein channels are also called TRAAK channels.

Acid-sensing ion channel 2 (ASIC2) also known as amiloride-sensitive cation channel 1, neuronal (ACCN1) or brain sodium channel 1 (BNaC1) is a protein that in humans is encoded by the ASIC2 gene. The ASIC2 gene is one of the five paralogous genes that encode proteins that form trimeric acid-sensing ion channels (ASICs) in mammals. The cDNA of this gene was first cloned in 1996. The ASIC genes have splicing variants that encode different proteins that are called isoforms.

Potassium voltage-gated channel subfamily B member 2 is a protein that in humans is encoded by the KCNB2 gene. The protein encoded by this gene is a voltage-gated potassium channel subunit.

Potassium voltage-gated channel subfamily V member 1 is a protein that in humans is encoded by the KCNV1 gene. The protein encoded by this gene is a voltage-gated potassium channel subunit.

Potassium channel, subfamily K, member 10, also known as KCNK10 is a human gene. The protein encoded by this gene, K2P10.1, is a potassium channel containing two pore-forming P domains.
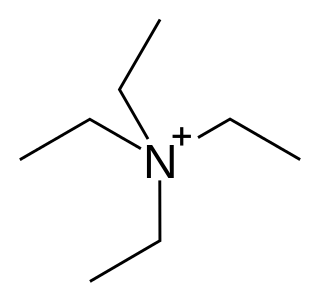
Potassium channel blockers are agents which interfere with conduction through potassium channels.

A channel blocker is the biological mechanism in which a particular molecule is used to prevent the opening of ion channels in order to produce a physiological response in a cell. Channel blocking is conducted by different types of molecules, such as cations, anions, amino acids, and other chemicals. These blockers act as ion channel antagonists, preventing the response that is normally provided by the opening of the channel.
A potassium channel opener is a type of drug which facilitates ion transmission through potassium channels.
Mechanosensitive channels (MSCs), mechanosensitive ion channels or stretch-gated ion channels are membrane proteins capable of responding to mechanical stress over a wide dynamic range of external mechanical stimuli. They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes.

Psalmotoxin (PcTx1) is a spider toxin from the venom of the Trinidad tarantula Psalmopoeus cambridgei. It selectively blocks Acid Sensing Ion Channel 1-a (ASIC1a), which is a proton-gated sodium channel.

Colin G. Nichols FRS is the Carl Cori Endowed Professor, and Director of the Center for Investigation of Membrane Excitability Diseases at Washington University in St. Louis, Missouri.
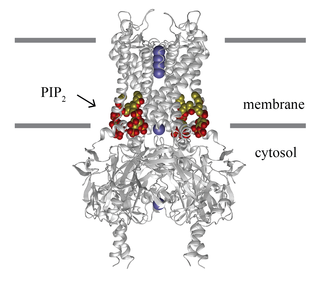
Lipid-gated ion channels are a class of ion channels whose conductance of ions through the membrane depends directly on lipids. Classically the lipids are membrane resident anionic signaling lipids that bind to the transmembrane domain on the inner leaflet of the plasma membrane with properties of a classic ligand. Other classes of lipid-gated channels include the mechanosensitive ion channels that respond to lipid tension, thickness, and hydrophobic mismatch. A lipid ligand differs from a lipid cofactor in that a ligand derives its function by dissociating from the channel while a cofactor typically derives its function by remaining bound.
References
- ↑ "Université côte d'Azur".
- ↑ "Académie des sciences".
- ↑ "Arrêté du 2 décembre 1991 portant nomination à l'Institut universitaire de France". Legifrance. Retrieved 8 March 2020.
- ↑ "Arrêté du 8 août 1996 portant nomination des membres seniors et juniors de l'Institut universitaire de France". Legifrance. Retrieved 8 March 2020.
- 1 2 3 4 5 "Michel Lazdunski-Research gate".
- 1 2 3 4 Michel Lazdunski publications indexed by Google Scholar
- ↑ Amoroso, Salvatore; Schmid-Antomarchi, Heidy; Fosset, Michel; Lazdunski, Michel (16 February 1990). "Glucose, Sulfonylureas, and Neurotransmitter Release: Role of ATP-Sensitive K + Channels". Science. 247 (4944). American Association for the Advancement of Science (AAAS): 852–854. Bibcode:1990Sci...247..852A. doi:10.1126/science.2305257. ISSN 0036-8075. PMID 2305257.
- ↑ Waldmann, Rainer; Champigny, Guy; Bassilana, Frédéric; Heurteaux, Catherine; Lazdunski, Michel (1997). "A proton-gated cation channel involved in acid-sensing". Nature. 386 (6621). Springer Science and Business Media LLC: 173–177. Bibcode:1997Natur.386..173W. doi:10.1038/386173a0. ISSN 0028-0836. PMID 9062189. S2CID 4361943.
- ↑ Heurteaux, Catherine; Lucas, Guillaume; Guy, Nicolas; El Yacoubi, Malika; Thümmler, Susanne; Peng, Xiao-Dong; Noble, Florence; Blondeau, Nicolas; Widmann, Catherine; Borsotto, Marc; Gobbi, Gabriella; Vaugeois, Jean-Marie; Debonnel, Guy; Lazdunski, Michel (13 August 2006). "Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype". Nature Neuroscience. 9 (9). Springer Science and Business Media LLC: 1134–1141. doi:10.1038/nn1749. ISSN 1097-6256. PMID 16906152. S2CID 22729624.
- ↑ Patel, Amanda J.; Honoré, Eric; Lesage, Florian; Fink, Michel; Romey, Georges; Lazdunski, Michel (1999). "Inhalational anesthetics activate two-pore-domain background K+ channels". Nature Neuroscience. 2 (5). Springer Science and Business Media LLC: 422–426. doi:10.1038/8084. ISSN 1097-6256. PMID 10321245. S2CID 23092576.
- ↑ Dalemans, Wilfried; Barbry, Pascal; Champigny, Guy; Jallat, Sophie; Jallat, Sophie; Dott, Karin; Dreyer, Dominique; Crystal, Ronald G.; Pavirani, Andréa; Lecocq, Jean-Pierre; Lazdunski, Michel (1991). "Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation". Nature. 354 (6354). Springer Science and Business Media LLC: 526–528. Bibcode:1991Natur.354..526D. doi: 10.1038/354526a0 . ISSN 0028-0836. PMID 1722027. S2CID 4233457.