Related Research Articles
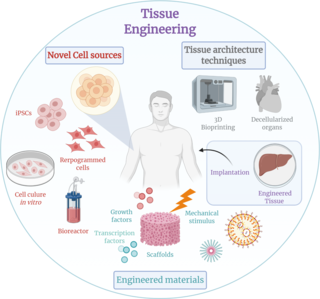
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can be considered as a field of its own.

A bioreactor is any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.
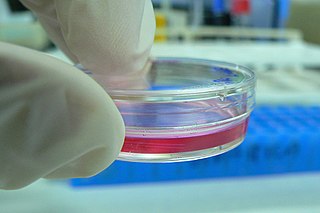
Cell culture or tissue culture is the process by which cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. They need to be kept at body temperature (37 °C) in an incubator. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or rich medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate to form an adherent culture as a monolayer (one single-cell thick), whereas others can be grown free floating in a medium as a suspension culture. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Tissue culture commonly refers to the culture of animal cells and tissues, with the more specific term plant tissue culture being used for plants. The lifespan of most cells is genetically determined, but some cell-culturing cells have been 'transformed' into immortal cells which will reproduce indefinitely if the optimal conditions are provided.

Chondrocytes are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes can differentiate into various cell types, including osteoblasts.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".
Stem-cell therapy uses stem cells to treat or prevent a disease or condition. As of 2024, the only FDA-approved therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone marrow or peripheral blood stem cell transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.
Articular cartilage, most notably that which is found in the knee joint, is generally characterized by very low friction, high wear resistance, and poor regenerative qualities. It is responsible for much of the compressive resistance and load bearing qualities of the knee joint and, without it, walking is painful to impossible. Osteoarthritis is a common condition of cartilage failure that can lead to limited range of motion, bone damage and invariably, pain. Due to a combination of acute stress and chronic fatigue, osteoarthritis directly manifests itself in a wearing away of the articular surface and, in extreme cases, bone can be exposed in the joint. Some additional examples of cartilage failure mechanisms include cellular matrix linkage rupture, chondrocyte protein synthesis inhibition, and chondrocyte apoptosis. There are several different repair options available for cartilage damage or failure.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
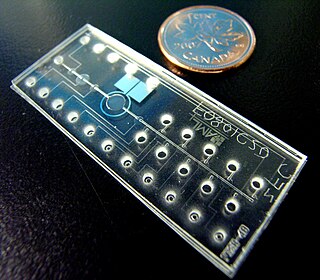
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.
Human platelet lysate is a substitute supplement for fetal bovine serum (FBS) in experimental and clinical cell culture. It is a turbid, light-yellow liquid that is obtained from human blood platelets after freeze/thaw cycle(s). The freeze/thaw cycle causes the platelets to lyse, releasing a large quantity of growth factors necessary for cell expansion. hPL has the highest concentration of growth factors of any serum supplements. FBS-free cell culture media, e.g. with platelet lysate or chemically defined/ animal component free, are used for cell therapy or regenerative medicine. They are commercially available in GMP -quality which is generally basis for regulatory approval.
Dermal fibroblasts are cells within the dermis layer of skin which are responsible for generating connective tissue and allowing the skin to recover from injury. Using organelles, dermal fibroblasts generate and maintain the connective tissue which unites separate cell layers. Furthermore, these dermal fibroblasts produce the protein molecules including laminin and fibronectin which comprise the extracellular matrix. By creating the extracellular matrix between the dermis and epidermis, fibroblasts allow the epithelial cells of the epidermis to affix the matrix, thereby allowing the epidermal cells to effectively join together to form the top layer of the skin.
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments, a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.
A Hollow fiber bioreactor is a 3 dimensional cell culturing system based on hollow fibers, which are small, semi-permeable capillary membranes arranged in parallel array with a typical molecular weight cut-off (MWCO) range of 10-30 kDa. These hollow fiber membranes are often bundled and housed within tubular polycarbonate shells to create hollow fiber bioreactor cartridges. Within the cartridges, which are also fitted with inlet and outlet ports, are two compartments: the intracapillary (IC) space within the hollow fibers, and the extracapillary (EC) space surrounding the hollow fibers.
Gelatin Microparticles are polymer microparticles constructed of gelatin. Gelatin, a bipolymer, is produced through the hydrolysis of collagen. Gelatin, along with its more familiar uses, is widely used for the production of microparticles due to its efficiency in forming gels as well as its biodegradability as a particle. Gelatin can be manipulated to form a stable matrix for biologically reactive compounds, allowing for the incorporation and protection against enzymatic degradation. Gelatin Microparticles are versatile particles and are easily loadable for the use within drug systems and alongside growth factors post-synthesis. Gelatin microparticles also allow for the biochemically controlled release of drug particles, growth factors, and other biological molecules.
The stem cell secretome is a collective term for the paracrine soluble factors produced by stem cells and utilized for their inter-cell communication. In addition to inter-cell communication, the paracrine factors are also responsible for tissue development, homeostasis and (re-)generation. The stem cell secretome consists of extracellular vesicles, specifically exosomes, microvesicles, membrane particles, peptides and small proteins (cytokines). The paracrine activity of stem cells, i.e. the stem cell secretome, has been found to be the predominant mechanism by which stem cell-based therapies mediate their effects in degenerative, auto-immune and/or inflammatory diseases. Though not only stem cells possess a secretome which influences their cellular environment, their secretome currently appears to be the most relevant for therapeutic use.

Air liquid interface cell culture (ALI) is a method of cell culture by which basal stem cells are grown with their basal surfaces in contact with media, and the top of the cellular layer is exposed to the air. The cells are then lifted and media is changed until the development of a mucociliary phenotype of a pseudostratified epithelium, similar to the tracheal epithelium.
Artificial cartilage is a synthetic material made of hydrogels or polymers that aims to mimic the functional properties of natural cartilage in the human body. Tissue engineering principles are used in order to create a non-degradable and biocompatible material that can replace cartilage. While creating a useful synthetic cartilage material, certain challenges need to be overcome. First, cartilage is an avascular structure in the body and therefore does not repair itself. This creates issues in regeneration of the tissue. Synthetic cartilage also needs to be stably attached to its underlying surface i.e. the bone. Lastly, in the case of creating synthetic cartilage to be used in joint spaces, high mechanical strength under compression needs to be an intrinsic property of the material.
Entomoculture is the subfield of cellular agriculture which specifically deals with the production of insect tissue in vitro. It draws on principles more generally used in tissue engineering and has scientific similarities to Baculovirus Expression Vectors or soft robotics. The field has mainly been proposed because of its potential technical advantages over mammalian cells in generating cultivated meat. The name of the field was coined by Natalie Rubio at Tufts University.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Petry F, Salzig D (2021). "Impact of Bioreactor Geometry on Mesenchymal Stem Cell Production in Stirred-Tank Bioreactors". Chemie Ingenieur Technik. 93 (10): 1537–1554. doi: 10.1002/cite.202100041 . ISSN 1522-2640. S2CID 238704820.
- 1 2 3 4 5 6 7 8 9 10 11 12 Chen AK, Reuveny S, Oh SK (November 2013). "Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction". Biotechnology Advances. Stem Cell Engineering. 31 (7): 1032–1046. doi: 10.1016/j.biotechadv.2013.03.006 . PMID 23531528.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Li B, Wang X, Wang Y, Gou W, Yuan X, Peng J, et al. (April 2015). "Past, present, and future of microcarrier-based tissue engineering". Journal of Orthopaedic Translation. 3 (2): 51–57. doi:10.1016/j.jot.2015.02.003. PMC 5982391 . PMID 30035040.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Tsai AC, Pacak CA (July 2021). "Bioprocessing of Human Mesenchymal Stem Cells: From Planar Culture to Microcarrier-Based Bioreactors". Bioengineering. 8 (7): 96. doi: 10.3390/bioengineering8070096 . PMC 8301102 . PMID 34356203.
- 1 2 3 Badenes SM, Fernandes TG, Rodrigues CA, Diogo MM, Cabral JM (September 2016). "Microcarrier-based platforms for in vitro expansion and differentiation of human pluripotent stem cells in bioreactor culture systems". Journal of Biotechnology. 234: 71–82. doi:10.1016/j.jbiotec.2016.07.023. PMID 27480342.
- 1 2 3 Rafiq QA, Coopman K, Nienow AW, Hewitt CJ (March 2016). "Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors". Biotechnology Journal. 11 (4): 473–486. doi:10.1002/biot.201400862. PMC 4991290 . PMID 26632496.
- ↑ Chen P, Luo Z, Güven S, Tasoglu S, Ganesan AV, Weng A, Demirci U (September 2014). "Microscale assembly directed by liquid-based template". Advanced Materials. 26 (34): 5936–41. doi:10.1002/adma.201402079. PMC 4159433 . PMID 24956442.