
Pterin is a heterocyclic compound composed of a pteridine ring system, with a "keto group" and an amino group on positions 4 and 2 respectively. It is structurally related to the parent bicyclic heterocycle called pteridine. Pterins, as a group, are compounds related to pterin with additional substituents. Pterin itself is of no biological significance.

Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor.

Sulfite oxidase is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts. It oxidizes sulfite to sulfate and, via cytochrome c, transfers the electrons produced to the electron transport chain, allowing generation of ATP in oxidative phosphorylation. This is the last step in the metabolism of sulfur-containing compounds and the sulfate is excreted.
Trimethylamine N-oxide reductase is a microbial enzyme that can reduce trimethylamine N-oxide (TMAO) into trimethylamine (TMA), as part of the electron transport chain. The enzyme has been purified from E. coli and the photosynthetic bacteria Roseobacter denitrificans.

Molybdenum cofactor synthesis protein 2A and molybdenum cofactor synthesis protein 2B are a pair of proteins that in humans are encoded from the same MOCS2 gene. These two proteins dimerize to form molybdopterin synthase.

Adenylyl cyclase type 8 is an enzyme that in humans is encoded by the ADCY8 gene.

Adenylyltransferase and sulfurtransferase MOCS3 is an enzyme that in humans is encoded by the MOCS3 gene.

Adenylyl cyclase 10 also known as ADCY10 is an enzyme that, in humans, is encoded by the ADCY10 gene.
Molybdopterin synthase (EC 2.8.1.12, MPT synthase) is an enzyme required to synthesize molybdopterin (MPT) from precursor Z (now known as cyclic pyranopterin monophosphate). Molydopterin is subsequently complexed with molybdenum to form molybdenum cofactor (MoCo). MPT synthase catalyses the following chemical reaction:
Lipoate–protein ligase (EC 2.7.7.63, LplA, lipoate protein ligase, lipoate–protein ligase A, LPL, LPL-B) is an enzyme with systematic name ATP:lipoate adenylyltransferase. This enzyme catalyses the following chemical reaction
Molybdopterin adenylyltransferase is an enzyme with systematic name ATP:molybdopterin adenylyltransferase. This enzyme catalyses the following chemical reaction
Molybdenum cofactor cytidylyltransferase is an enzyme with systematic name CTP:molybdenum cofactor cytidylyltransferase. This enzyme catalyses the following chemical reaction:
Molybdenum cofactor guanylyltransferase is an enzyme with systematic name GTP:molybdenum cofactor guanylyltransferase. This enzyme catalyses the following chemical reaction:
Molybdopterin-synthase adenylyltransferase is an enzyme with systematic name ATP:molybdopterin-synthase adenylyltransferase. This enzyme catalyses the following chemical reaction
Molybdenum cofactor sulfurtransferase (EC 2.8.1.9, molybdenum cofactor sulfurase, ABA3, MoCo sulfurase, MoCo sulfurtransferase) is an enzyme with systematic name L-cysteine:molybdenum cofactor sulfurtransferase. This enzyme catalyses the following chemical reaction
Molybdopterin synthase sulfurtransferase is an enzyme with systematic name persulfurated L-cysteine desulfurase:(molybdopterin-synthase sulfur-carrier protein)-Gly-Gly sulfurtransferase. This enzyme catalyses the following chemical reaction
Cyclic pyranopterin monophosphate synthase is an enzyme with systematic name GTP 8,9-lyase . This enzyme catalyses the following chemical reaction

In enzymology, an aldehyde ferredoxin oxidoreductase (EC 1.2.7.5) is an enzyme that catalyzes the chemical reaction

Mitochondrial amidoxime-reducing component 1 is a mammalian molybdenum-containing enzyme. It is located in the outer mitochondrial membrane and consists of a N-terminal mitochondrial signal domain facing the inter-membrane space, a transmembrane domain, and a C-terminal catalytic domain facing the cytosol. In humans it is encoded by the MOSC1 gene.
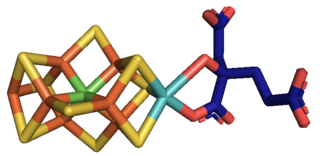
Molybdenum is an essential element in most organisms. It is most notably present in nitrogenase which is an essential part of nitrogen fixation.









