
Aconitine is an alkaloid toxin produced by various plant species belonging to the genus Aconitum, commonly known by the names wolfsbane and monkshood. Aconitine is notorious for its toxic properties.

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.
A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.
Fluoride toxicity is a condition in which there are elevated levels of the fluoride ion in the body. Although fluoride is safe for dental health at low concentrations, sustained consumption of large amounts of soluble fluoride salts is dangerous. Referring to a common salt of fluoride, sodium fluoride (NaF), the lethal dose for most adult humans is estimated at 5 to 10 g. Ingestion of fluoride can produce gastrointestinal discomfort at doses at least 15 to 20 times lower than lethal doses. Although it is helpful topically for dental health in low dosage, chronic ingestion of fluoride in large amounts interferes with bone formation. In this way, the most widespread examples of fluoride poisoning arise from consumption of ground water that is abnormally fluoride-rich.

T-2 mycotoxin is a trichothecene mycotoxin. It is a naturally occurring mold byproduct of Fusarium spp. fungus which is toxic to humans and other animals. The clinical condition it causes is alimentary toxic aleukia and a host of symptoms related to organs as diverse as the skin, airway, and stomach. Ingestion may come from consumption of moldy whole grains. T-2 can be absorbed through human skin. Although no significant systemic effects are expected after dermal contact in normal agricultural or residential environments, local skin effects can not be excluded. Hence, skin contact with T-2 should be limited.

Fumonisin B1 is the most prevalent member of a family of toxins, known as fumonisins, produced by multiple species of Fusarium molds, such as Fusarium verticillioides, which occur mainly in maize (corn), wheat and other cereals. Fumonisin B1 contamination of maize has been reported worldwide at mg/kg levels. Human exposure occurs at levels of micrograms to milligrams per day and is greatest in regions where maize products are the dietary staple.

The trichothecenes are a large family of chemically related mycotoxins. They are produced by various species of Fusarium, Myrothecium, Trichoderma/Podostroma, Trichothecium, Cephalosporium, Verticimonosporium, and Stachybotrys. Chemically, trichothecenes are a class of sesquiterpenes.

Zearalenone (ZEN), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some Fusarium and Gibberella species. Specifically, the Gibberella zeae, the fungal species where zearalenone was initially detected, in its asexual/anamorph stage is known as Fusarium graminearum. Several Fusarium species produce toxic substances of considerable concern to livestock and poultry producers, namely deoxynivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) and zearalenone. Particularly, ZEN is produced by Fusarium graminearum, Fusarium culmorum, Fusarium cerealis, Fusarium equiseti, Fusarium verticillioides, and Fusarium incarnatum. Zearalenone is the primary toxin that binds to estrogen receptors, causing infertility, abortion or other breeding problems, especially in swine. Often, ZEN is detected together with deoxynivalenol in contaminated samples and its toxicity needs to be considered in combination with the presence of other toxins.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it can cause a variety of toxic effects, including kidney, liver and cell damage. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Methyllycaconitine was identified one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralysis, and it has been shown to have insecticidal properties. It has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.
Mycotoxicology is the branch of mycology that focuses on analyzing and studying the toxins produced by fungi, known as mycotoxins. In the food industry it is important to adopt measures that keep mycotoxin levels as low as practicable, especially those that are heat-stable. These chemical compounds are the result of secondary metabolism initiated in response to specific developmental or environmental signals. This includes biological stress from the environment, such as lower nutrients or competition for those available. Under this secondary path the fungus produces a wide array of compounds in order to gain some level of advantage, such as incrementing the efficiency of metabolic processes to gain more energy from less food, or attacking other microorganisms and being able to use their remains as a food source.
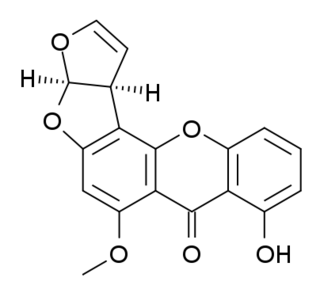
Sterigmatocystin is a polyketide mycotoxin produced by certain species of Aspergillus. The toxin is naturally found in some cheeses.

Vomitoxin, also known as deoxynivalenol (DON), is a type B trichothecene, an epoxy-sesquiterpenoid. This mycotoxin occurs predominantly in grains such as wheat, barley, oats, rye, and corn, and less often in rice, sorghum, and triticale. The occurrence of deoxynivalenol is associated primarily with Fusarium graminearum and F. culmorum, both of which are important plant pathogens which cause fusarium head blight in wheat and gibberella or fusarium ear blight in corn. The incidence of fusarium head blight is strongly associated with moisture at the time of flowering (anthesis), and the timing of rainfall, rather than the amount, is the most critical factor. However, increased amount of moisture towards harvest time has been associated with lower amount of vomitoxin in wheat grain due to leaching of toxins. Furthermore, deoxynivalenol contents are significantly affected by the susceptibility of cultivars towards Fusarium species, previous crop, tillage practices, and fungicide use. It occurs abundantly in grains in Norway due to heavy rainfall.
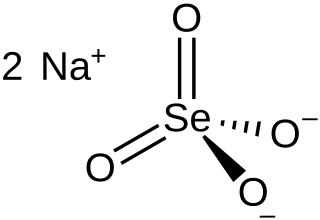
Sodium selenate is the inorganic compound with the formula Na
2SeO
4. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor.

Aflatoxin B1 is an aflatoxin produced by Aspergillus flavus and A. parasiticus. It is a very potent carcinogen with a TD50 3.2 μg/kg/day in rats. This carcinogenic potency varies across species with some, such as rats and monkeys, seemingly much more susceptible than others. Aflatoxin B1 is a common contaminant in a variety of foods including peanuts, cottonseed meal, corn, and other grains; as well as animal feeds. Aflatoxin B1 is considered the most toxic aflatoxin and it is highly implicated in hepatocellular carcinoma (HCC) in humans. In animals, aflatoxin B1 has also been shown to be mutagenic, teratogenic, and to cause immunosuppression. Several sampling and analytical methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry, and enzyme-linked immunosorbent assay (ELISA), among others, have been used to test for aflatoxin B1 contamination in foods. According to the Food and Agriculture Organization (FAO), a division of the United Nations, the worldwide maximum tolerated levels of aflatoxin B1 was reported to be in the range of 1–20 μg/kg (or .001 ppm - 1 part-per-billion) in food, and 5–50 μg/kg (.005 ppm) in dietary cattle feed in 2003.

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.

Nivalenol (NIV) is a mycotoxin of the trichothecene group. In nature it is mainly found in fungi of the Fusarium species. The Fusarium species belongs to the most prevalent mycotoxin producing fungi in the temperate regions of the northern hemisphere, therefore making them a considerable risk for the food crop production industry.

Penicillin Roquefort toxin is a mycotoxin produced by the fungus Penicillium roqueforti. In 1973, PR toxin was first partially characterized by isolating moldy corn on which the fungi had grown. Although its lethal dose was determined shortly after the isolation of the chemical, details of its toxic effects were not fully clarified until 1982 in a study with mice, rats, anesthetized cats and preparations of isolated rat auricles.
















