
A carcinogen is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division.

In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes.
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.
Antihypertensives are a class of drugs that are used to treat hypertension. Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke, heart failure, kidney failure and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, ACE inhibitors, angiotensin II receptor antagonists (ARBs), and beta blockers.

Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system.
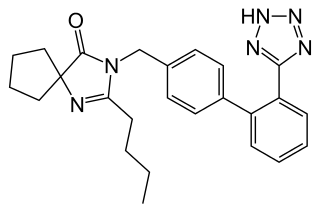
Irbesartan, sold under the brand name Avapro among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versions are available as the combination irbesartan/hydrochlorothiazide.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.
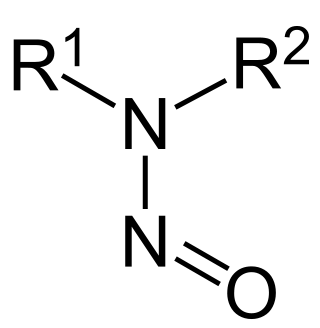
In organic chemistry, nitrosamines are organic compounds with the chemical structure R2N−N=O, where R is usually an alkyl group. They feature a nitroso group bonded to a deprotonated amine. Most nitrosamines are carcinogenic in nonhuman animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive".

Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besides hypertension, it is also used in diabetic kidney disease, heart failure, and left ventricular enlargement. It comes as a tablet that is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.
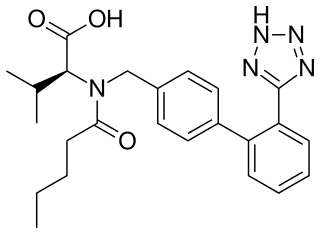
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth.

Nicotine-derived nitrosamine ketone (NNK) is one of the key tobacco-specific nitrosamines derived from nicotine. It plays an important role in carcinogenesis. The conversion of nicotine to NNK entails opening of the pyrrolidine ring.
Torrent Pharmaceuticals Ltd is an Indian multinational pharmaceutical company, part of the Torrent Group and headquartered in Ahmedabad. It was promoted by U. N. Mehta, initially as Trinity Laboratories Ltd, and was later renamed Torrent Pharmaceuticals Ltd.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure. They are especially useful in quantifying an organism's exposure to a carcinogen. The presence of such an adduct indicates prior exposure to a potential carcinogen, but it does not necessarily indicate the presence of cancer in the subject animal.

N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals.
The angiotensin receptor blockers (ARBs), also called angiotensin (AT1) receptor antagonists or sartans, are a group of antihypertensive drugs that act by blocking the effects of the hormone angiotensin II in the body, thereby lowering blood pressure. Their structure is similar to Ang II and they bind to Ang II receptors as inhibitors, e.g., [T24 from Rhys Healthcare].

Valsartan/hydrochlorothiazide, sold under the brand name Diovan HCT among others, is a medication used to treat high blood pressure when valsartan is not sufficient. It is a combination of valsartan, an angiotensin receptor blocker with hydrochlorothiazide, a diuretic. It is taken by mouth.

Aurobindo Pharma Limited is an Indian multinational pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad, India. The company manufactures generic pharmaceuticals and active pharmaceutical ingredients. The company’s area of activity includes six major therapeutic and product areas: antibiotics, anti-retrovirals, cardiovascular products, central nervous system products, gastroenterologicals, and anti-allergics. The company markets these products in over 125 countries. Its marketing partners include AstraZeneca and Pfizer.
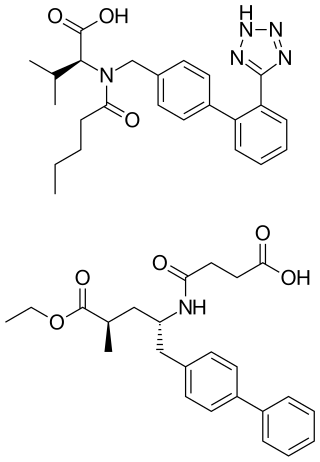
Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi). In 2016, the American College of Cardiology/American Heart Association Task Force recommended it as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.
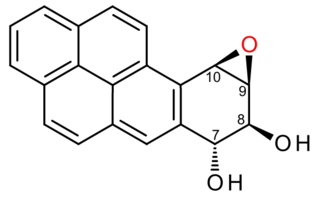
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.
Additive effect in pharmacology describes the situation when the combining effects of two drugs equal the sum of the effects of the two drugs acting independently. The concept of additive effect is derived from the concept of synergy. It was introduced by the scientists in pharmacology and biochemistry fields in the process of understanding the synergistic interaction between drugs and chemicals over the century.















