
Alfred Bernhard Nobel was a Swedish chemist, inventor, engineer and businessman. He is known for inventing dynamite as well as having bequeathed his fortune to establish the Nobel Prizes. He also made several important contributions to science, holding 355 patents in his lifetime.

Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices.

Rayon, also called viscose and commercialised in some countries as sabra silk or cactus silk, is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk. It can be woven or knit to make textiles for clothing and other purposes.
Twaron is a para-aramid, high-performance yarn. It is a heat-resistant fibre, helps in ballistic protection and cut protection. Twaron was developed in the early 1970s by the Dutch company Akzo Nobel's division Enka BV, later Akzo Industrial Fibers. The research name of the para-aramid fibre was originally Fiber X, but it was soon called Arenka. Although the Dutch para-aramid fiber was developed only a little later than DuPont's Kevlar, the introduction of Twaron as a commercial product came much later than Kevlar due to financial problems at the Akzo company in the 1970s. As of 2000, Twaron had become a global material and had been integrated into the global markets. Twaron has been around for over 30 years.

1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.

Akzo Nobel N.V., stylised as AkzoNobel, is a Dutch multinational company which creates paints and performance coatings for both industry and consumers worldwide. Headquartered in Amsterdam, the company has activities in more than 150 countries. AkzoNobel is the world's third-largest paint manufacturer by revenue after Sherwin-Williams and PPG Industries.

PPG Industries, Inc. is an American Fortune 500 company and global supplier of paints, coatings, and specialty materials. With headquarters in Pittsburgh, Pennsylvania, PPG operates in more than 70 countries around the globe. By revenue it is the largest coatings company in the world followed by Sherwin-Williams. It is headquartered in PPG Place, an office and retail complex in downtown Pittsburgh, and is known for its glass facade designed by Postmodern architect Philip Johnson.

Methyl isobutyl ketone (MIBK, 4-methylpentan-2-one) is an organic compound with the condensed chemical formula (CH3)2CHCH2C(O)CH3. This ketone is a colourless liquid that is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose.

Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.

Dulux is an internationally-available brand of architectural paint that originated from the United Kingdom. The brand name Dulux has been used by both Imperial Chemical Industries (ICI) and DuPont since 1931 and was one of the first alkyd-based paints. It is produced by AkzoNobel although the North American market is now served by PPG Industries.
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.
Organon & Co. is an American pharmaceutical company headquartered in Jersey City, New Jersey. Organon specializes in the following core therapeutic fields: reproductive medicine, contraception, psychiatry, hormone replacement therapy (HRT), and anesthesia. Organon produces all its products outside of the United States but gets a third of its revenue from the United States.
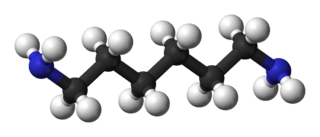
Hexamethylenediamine or hexane-1,6-diamine, is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.
The alkyne zipper reaction is an organic reaction that involves isomerization of a non terminal alkyne into a terminal alkyne. This reaction was first reported by Alexey Favorsky in 1887. Also, this reaction was reported by Charles Allen Brown and Ayako Yamashita in 1975. The isomerization reaction proceeds for straight-chain alkynes and acetylinic alcohols. The conversion provides a useful approach for remote functionalization in long-chain alkynes.

International Paint, abbreviated as International, is a brand of the Marine & Protective Coatings business unit of AkzoNobel.

Glidden is an American paint brand, manufactured by PPG Industries.

Isophorone diamine (usually shortened to IPDA) is a chemical compound and specifically a diamine with the formula (CH3)3C6H7(NH2)(CH2NH2). It is a colorless liquid. It is a precursor to polymers and coatings.

Salpn is the common name for a chelating ligand, properly called N,N′-bis(salicylidene)-1,2-propanediamine, used as a motor oil additive.

1,2-Diaminocyclohexane (DACH) is an organic compound with the formula (CH2)4(CHNH2)2. It is a mixture of three stereoisomers: cis-1,2-diaminocyclohexane and both enantiomers of trans-1,2-diaminocyclohexane. The mixture is a colorless, corrosive liquid, although older samples can appear yellow. It is often called DCH-99 and also DACH.

Laurylamine Dipropylenediamine (DPTA) is an organic compound in the class of dodecylamines. It appears as a colourless or yellow liquid with an amine-like odour.






















