
Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.
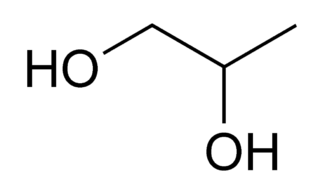
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.

Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless, pyrophoric gas with a repulsively sweet odor. Synonyms include boroethane, boron hydride, and diboron hexahydride. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Its derivatives are useful reagents.

Ethylene oxide is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
Brake fluid is a type of hydraulic fluid used in hydraulic brake and hydraulic clutch applications in automobiles, motorcycles, light trucks, and some bicycles. It is used to transfer force into pressure, and to amplify braking force. It works because liquids are not appreciably compressible.

Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water.
The National Emission Standards for Hazardous Air Pollutants, also using the acronym NESHAP, are emission standards set by the United States Environmental Protection Agency—EPA. The standards are for air pollutants not covered by National Ambient Air Quality Standards—NAAQS, that may cause an increase in fatalities or in serious, irreversible, or incapacitating illness.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction, the study also reported a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.

Diethylene glycol (DEG) is an organic compound with the formula (HOCH2CH2)2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is a four carbon dimer of ethylene glycol. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent. It can be a contaminant in consumer products; this has resulted in numerous epidemics of poisoning since the early 20th century.

Ethylene glycol dinitrate, abbreviated EGDN and NGc, also known as nitroglycol, is a chemical compound a colorless, oily explosive liquid obtained by nitrating ethylene glycol. It is similar to nitroglycerin in both manufacture and properties, though it is more volatile and less viscous. Unlike nitroglycerine, the chemical has a perfect (0) oxygen balance, meaning that its ideal exothermic decomposition would completely convert it to low energy CO2, H2O, and N2, with no excess unreacted O2, H2, C, or other high-energy substances, without needing to react with anything else.
Glycol ethers are a group of solvents based on alkyl ethers of ethylene glycol or propylene glycol commonly used in paints and cleaners. These solvents typically have a higher boiling point, together with the favorable solvent properties of lower-molecular weight ethers and alcohols. The word "Cellosolve" was registered in 1924 as a United States trademark by Carbide & Carbon Chemicals Corp. for "Solvents for Gums, Resins, Cellulose Esters, and the Like". The first one was ethyl cellosolve, with the name now generic for glycol ethers.

Diglyme, or bis(2-methoxyethyl) ether, is a solvent with a high boiling point. It is an organic compound which is the dimethyl ether of diethylene glycol. It is a colorless liquid with a slight ether-like odor. It is miscible with water as well as organic solvents.
Pentaethylene glycol monododecyl ether (C12E5) is a nonionic surfactant formed by the ethoxylation of dodecanol to give a material with 5 repeat units of ethylene glycol.
Nonoxynols also known as nonaethylene glycol or polyethylene glycol nonyl phenyl ether are mixtures of nonionic surfactants used as detergents, emulsifiers, wetting agents or defoaming agents. The most commonly discussed compound nonoxynol-9 is a spermicide, formulated primarily as a component of vaginal foams and creams. Nonoxynol was found to metabolize into free nonylphenol when administered to lab animals. Arkopal-N60, with on average 6 ethylene glycol units is a related used surfactant.
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable chemical compound (chemical formula CH3CHOHCH3) with a strong odor. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.

Propylene glycol methyl ether is an organic solvent with a wide variety of industrial and commercial uses. Similar to other glycol ethers, it is used as a carrier/solvent in printing/writing inks and paints/coatings. It also finds use as an industrial and commercial paint stripper. It is used as an antifreeze in diesel engines.












