In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Lactones are cyclic carboxylic esters are intramolecular esters derived from hydroxy carboxylic acids. They can be saturated or unsaturated. Some contain heteroatoms replacing one or more carbon atoms of the ring.
1-Pentanol,, is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol. It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols, the undesirable byproducts of alcoholic fermentation.

Vinyl alcohol, also called ethenol or ethylenol, is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
Myristic acid is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. The name of the acyl group derived from myristic acid is myristoyl or tetradecanoyl. The acid is named after the binomial name for nutmeg, from which it was first isolated in 1841 by Lyon Playfair.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.
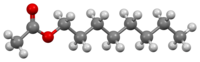
Capric acid, also known as decanoic acid or decylic acid, is a saturated fatty acid, medium-chain fatty acid (MCFA), and carboxylic acid. Its formula is CH3(CH2)8COOH. Salts and esters of decanoic acid are called caprates or decanoates. The term capric acid is derived from the Latin "caper / capra" (goat) because the sweaty, unpleasant smell of the compound is reminiscent of goats.

n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent.

Isoamyl acetate, also known as isopentyl acetate, is an ester formed from isoamyl alcohol and acetic acid, with the molecular formula . It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear. Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil or pear oil.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.
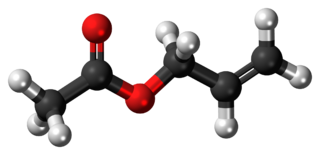
Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol.
Tridecylic acid, or tridecanoic acid, is the organic compound with the formula CH3(CH2)11CO2H. It is a 13-carbon saturated fatty acid. It is a white solid.

1-Octanol, also known as octan-1-ol, is the organic compound with the molecular formula CH3(CH2)7OH. It is a fatty alcohol. Many other isomers are also known generically as octanols. 1-Octanol is manufactured for the synthesis of esters for use in perfumes and flavorings. It has a pungent odor. Esters of octanol, such as octyl acetate, occur as components of essential oils. It is used to evaluate the lipophilicity of pharmaceutical products.

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.

2-Octanol is an organic compound with the chemical formula CH3CH(OH)(CH2)5CH3. It is a colorless oily liquid that is poorly soluble in water but soluble in most organic solvents. 2-Octanol is classified fatty alcohol. A secondary alcohol, it is chiral.
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. A colorless liquid at room temperature, it has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.
In industrial chemistry, carboalkoxylation is a process for converting alkenes to esters. This reaction is a form of carbonylation. A closely related reaction is hydrocarboxylation, which employs water in place of alcohols