Related Research Articles
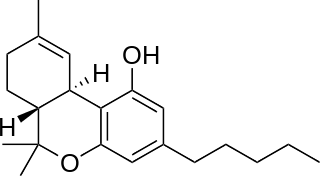
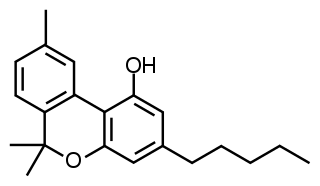
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the Delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. THC is a terpenoid found in cannabis and, like many pharmacologically active phytochemicals, it is assumed to be involved in the plant's evolutionary adaptation against insect predation, ultraviolet light, and environmental stress. THC was first discovered and isolated by Israeli chemist Raphael Mechoulam in Israel in 1964. It was found that, when smoked, THC is absorbed into the bloodstream and travels to the brain, attaching itself to endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia. These are the parts of the brain responsible for thinking, memory, pleasure, coordination and movement.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).

Tetrahydrocannabivarin is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of pentyl (5-carbon), making it non-psychoactive in lower doses. It has been shown to exhibit neuroprotective activity, appetite suppression, glycemic control and reduced side effects compared to THC, making it a potential treatment for management of obesity and diabetes.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis. Cannabigerol is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized.

The enzyme 1,4-dihydroxy-2-naphthoyl-CoA synthase catalyzes the sixth step in the biosynthesis of phylloquinone and menaquinone, the two forms of vitamin K. In E. coli, 1,4-dihydroxy-2-naphthoyl-CoA synthase, formerly known as naphthoate synthase, is encoded by menB and uses O-succinylbenzoyl-CoA as a substrate and converts it to 1,4-dihydroxy-2-naphthoyl-CoA.
The enzyme orsellinate decarboxylase (EC 4.1.1.58) catalyzes the chemical reaction

Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, is an anti-inflammatory which may contribute to the pain-killing effect of cannabis. It is one of the hundreds of cannabinoids found in the Cannabis plant, and is therefore a phytocannabinoid. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and its derivatives are as abundant as cannabinols in cannabis. It is not scheduled by the Convention on Psychotropic Substances. It is more common in tropical cannabis varieties.

Olivetol, also known as 5-pentylresorcinol or 5-pentyl-1,3-benzenediol, is an organic compound found in certain species of lichen; it is also a precursor in various syntheses of tetrahydrocannabinol.

Tetrahydrocannabinolic acid is a precursor of tetrahydrocannabinol (THC), an active component of cannabis.

Umbellic acid is a hydroxycinnamic acid. It is an isomer of caffeic acid.

Tetrahydrocannabinolic acid (THCA) synthase is an enzyme responsible for catalyzing the formation of THCA from cannabigerolic acid (CBGA). THCA is the direct precursor of tetrahydrocannabinol (THC), the principal psychoactive component of cannabis, which is produced from various strains of Cannabis sativa. Therefore, THCA synthase is considered to be a key enzyme controlling cannabis psychoactivity. Polymorphisms of THCA synthase result in varying levels of THC in Cannabis plants, resulting in "drug-type" and "fiber-type" C. sativa varieties.
Cannabidiolic acid synthase is an enzyme with systematic name cannabigerolate:oxygen oxidoreductase . It is an oxidoreductase found in Cannabis sativa that catalyses the formation of cannabidiolate, a carboxylated precursor of cannabidiol.
3,5,7-Trioxododecanoyl-CoA synthase (EC 2.3.1.206, TKS) is an enzyme with systematic name malonyl-CoA:hexanoyl-CoA malonyltransferase (3,5,7-trioxododecanoyl-CoA-forming). This enzyme catalyses the following chemical reaction
2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one 2-D-glucosyltransferase is an enzyme with systematic name UDP-alpha-D-glucose:2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one 2-beta-D-glucosyltransferase. This enzyme catalyses the following chemical reaction
Geranyl-pyrophosphate—olivetolic acid geranyltransferase is an enzyme with systematic name geranyl-diphosphate:olivetolate geranyltransferase. This enzyme catalyses the following chemical reaction

Olivetolic acid is an organic compound that is an intermediate in the biosynthetic pathway of the cannabinoids in Cannabis sativa.

Cannabitriol ((+)-CBT, (S,S)-9,10-Dihydroxy-Δ6a(10a)-THC) is a phytocannabinoid first isolated in 1966, an oxidation product of tetrahydrocannabinol which has been identified both as a trace component of cannabis and as a metabolite in cannabis users. Its pharmacology has been little studied, though it has been found to act as an antiestrogen and aromatase inhibitor.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.
References
- ↑ Gagne SJ, Stout JM, Liu E, Boubakir Z, Clark SM, Page JE (July 2012). "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides". Proceedings of the National Academy of Sciences of the United States of America. 109 (31): 12811–6. Bibcode:2012PNAS..10912811G. doi: 10.1073/pnas.1200330109 . PMC 3411943 . PMID 22802619.