
The ovary is a gonad in the female reproductive system that produces ova. When an ovum is released, this travels down the fallopian tube into the uterus. There is an ovary found on the left and the right side of the body. The ovaries also secrete hormones that play a role in the menstrual cycle and fertility. The ovary progresses through many stages beginning in the prenatal period through menopause. It is also an endocrine gland because of the various hormones that it secretes.
Cryobiology is the branch of biology that studies the effects of low temperatures on living things within Earth's cryosphere or in science. The word cryobiology is derived from the Greek words κρῧος [kryos], "cold", βίος [bios], "life", and λόγος [logos], "word". In practice, cryobiology is the study of biological material or systems at temperatures below normal. Materials or systems studied may include proteins, cells, tissues, organs, or whole organisms. Temperatures may range from moderately hypothermic conditions to cryogenic temperatures.

An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. At the time of puberty, women have approximately 200,000 to 300,000 follicles, each with the potential to release an egg cell (ovum) at ovulation for fertilization. These eggs are developed once every menstrual cycle with around 450–500 being ovulated during a woman's reproductive lifetime.
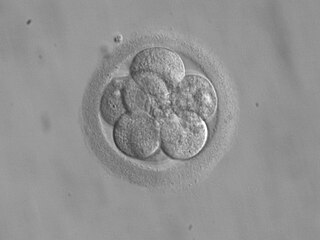
Embryo transfer refers to a step in the process of assisted reproduction in which embryos are placed into the uterus of a female with the intent to establish a pregnancy. This technique - which is often used in connection with in vitro fertilization (IVF) - may be used in humans or in other animals, in which situations and goals may vary.

Gynecologic ultrasonography or gynecologic sonography refers to the application of medical ultrasonography to the female pelvic organs as well as the bladder, the adnexa, and the recto-uterine pouch. The procedure may lead to other medically relevant findings in the pelvis.This technique is useful to detect myomas or mullerian malformations.

Growth/differentiation factor 9 is a protein that in humans is encoded by the GDF9 gene.
Primary ovarian insufficiency (POI), also called premature ovarian insufficiency, premature menopause, and premature ovarian failure, is the partial or total loss of reproductive and hormonal function of the ovaries before age 40 because of follicular dysfunction or early loss of eggs. POI can be seen as part of a continuum of changes leading to menopause that differ from age-appropriate menopause in the age of onset, degree of symptoms, and sporadic return to normal ovarian function. POI affects approximately 1 in 10,000 women under age 20, 1 in 1,000 women under age 30, and 1 in 100 of those under age 40. A medical triad for the diagnosis is amenorrhea, hypergonadotropism, and hypoestrogenism.

Oocyte cryopreservation is a procedure to preserve a woman's eggs (oocytes). This technique has been used to enable women to postpone pregnancy to a later date – whether for medical or social reasons. Several studies have shown that most infertility problems are due to germ cell deterioration related to aging. The procedure intends that the woman may choose to have the eggs thawed, fertilized, and transferred to the uterus as embryos to facilitate a pregnancy in the future. The procedure's success rate varies depending on the age of the woman, with the odds being higher in younger, adult women.

In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.
Poor ovarian reserve is a condition of low fertility characterized by 1): low numbers of remaining oocytes in the ovaries or 2) possibly impaired preantral oocyte development or recruitment. Recent research suggests that premature ovarian aging and premature ovarian failure may represent a continuum of premature ovarian senescence. It is usually accompanied by high FSH levels.
Fertility preservation is the effort to help cancer patients retain their fertility, or ability to procreate. Research into how cancer, ageing and other health conditions effect reproductive health and preservation options are growing. Specifically sparked in part by the increase in the survival rate of cancer patients.

Cryopreservation or cryoconservation is a process where biological material - cells, tissues, or organs - are frozen to preserve the material for an extended period of time. At low temperatures any cell metabolism which might cause damage to the biological material in question is effectively stopped. Cryopreservation is an effective way to transport biological samples over long distances, store samples for prolonged periods of time, and create a bank of samples for users. Molecules, referred to as cryoprotective agents (CPAs), are added to reduce the osmotic shock and physical stresses cells undergo in the freezing process. Some cryoprotective agents used in research are inspired by plants and animals in nature that have unique cold tolerance to survive harsh winters, including: trees, wood frogs, and tardigrades.
Semen cryopreservation is a procedure to preserve sperm cells. Semen can be used successfully indefinitely after cryopreservation. It can be used for sperm donation where the recipient wants the treatment in a different time or place, or as a means of preserving fertility for men undergoing vasectomy or treatments that may compromise their fertility, such as chemotherapy, radiation therapy or surgery. It is also often used by trans women prior to medically transitioning in ways that affect fertility, such as feminizing hormone therapy and orchiectomies.
Cryopreservation of embryos is the process of preserving an embryo at sub-zero temperatures, generally at an embryogenesis stage corresponding to pre-implantation, that is, from fertilisation to the blastocyst stage.
The Fertiprotekt network is a cooperation of university centres, hospitals and practices. It was founded in Germany in 2006. The network now extends to all German-speaking countries and currently units ca. 100 institutions in Germany, Austria and Switzerland.

Roger Gordon Gosden is a British-American physiologist in the field of female reproductive medicine. His scientific research focused on understanding the basic biology of development and senescence of ovaries in women, including mathematically modeling those processes. He did important translational research on ovarian tissue cryopreservation and transplantation.
Ovarian follicle activation can be defined as primordial follicles in the ovary moving from a quiescent (inactive) to a growing phase. The primordial follicle in the ovary is what makes up the “pool” of follicles that will be induced to enter growth and developmental changes that change them into pre-ovulatory follicles, ready to be released during ovulation. The process of development from a primordial follicle to a pre-ovulatory follicle is called folliculogenesis.

Evelyn Elizabeth Telfer is a reproductive biologist and professor at the University of Edinburgh. She leads a research team which has successfully grown immature human eggs to maturity in the lab, and discovered that human ovaries are capable of growing new eggs. In 2018 she was named one of Porter magazine's Incredible Women of 2018. In January 2019 she delivered the Anne McLaren Memorial Lecture at the Joint Fertility Societies Meeting in Birmingham: Fertility 2019. The Society of Reproduction and Fertility (SRF) presented her with their Distinguished Scientist award. Professor Telfer was presented with the Marshall Medal by SRF at Fertility 2023 in Belfast in recognition of her world leading contributions to the field of ovarian function and fertility preservation. The Marshall Medal is the Society’s premier award established in 1963 to commemorate the life and work of the eminent physiologist FHA Marshall.

An artificial ovary is a potential fertility preservation treatment that aims to mimic the function of the natural ovary.

LGBT reproduction refers to lesbian, gay, bisexual, and transgender (LGBT) people having biological children by means of assisted reproductive technology. It is distinct from LGBT parenting, which is a broader cultural phenomenon including LGBT adoption. In recent decades, developmental biologists have been researching and developing techniques to facilitate same-sex reproduction.













