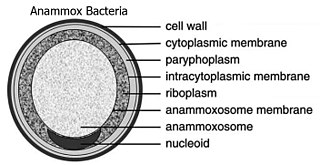
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammonium oxidation, also known as anammox. Many Planctomycetota occur in relatively high abundance as biofilms, often associating with other organisms such as macroalgae and marine sponges.

Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as Squalus is a genus of sharks). An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized derivatives such as bacteriohopanepolyols (BHPs) and hopanoids covalently attached to lipid A.
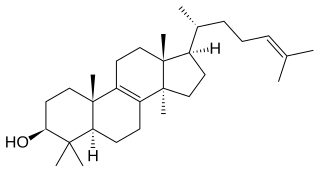
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast plant steroids are produced via cycloartenol.

Fatty acid synthase (FAS) is an enzyme that in humans is encoded by the FASN gene.
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.
(S)-2,3-Oxidosqualene ((S)-2,3-epoxysqualene) is an intermediate in the synthesis of the cell membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed when squalene is oxidized by the enzyme squalene monooxygenase. 2,3-Oxidosqualene is the substrate of various oxidosqualene cyclases, including lanosterol synthase, which produces lanosterol, a precursor to cholesterol.

Lanosterol synthase is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.
The enzyme sterol esterase (EC 3.1.1.13) catalyzes the reaction

In molecular biology, this protein domain belongs to the terpene synthase family (TPS). Its role is to synthesize terpenes, which are part of primary metabolism, such as sterols and carotene, and also part of the secondary metabolism. This entry will focus on the N terminal domain of the TPS protein.

Squalene-hopene cyclase (SHC) (EC 5.4.99.17) or hopan-22-ol hydro-lyase is an enzyme in the terpene cyclase/mutase family. It catalyzes the interconversion of squalene into a pentacyclic triterpenes, hopene and hopanol. This enzyme catalyses the following chemical reactions.
Lupeol synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
Achilleol B synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction

Gemmata obscuriglobus is a species of Gram-negative, aerobic, heterotrophic bacteria of the phylum Planctomycetota. G. obscuriglobus occur in freshwater habitats and was first described in 1984, and is the only described species in its genus.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.

Tetrahymanol is a gammacerane-type membrane lipid first found in the marine ciliate Tetrahymena pyriformis. It was later found in other ciliates, fungi, ferns, and bacteria. After being deposited in sediments that compress into sedimentary rocks over millions of years, tetrahymanol is dehydroxylated into gammacerane. Gammacerane has been interpreted as a proxy for ancient water column stratification.

Isoarborinol is a triterpenoid ubiquitously produced by angiosperms and is thus considered a biomarker for higher plants. Though no isoarborinol-producing microbe has been identified, isoarborinol is also considered a possible biomarker for marine bacteria, as its diagenetic end product, arborane, has been found in ancient marine sediments that predate the rise of plants. Importantly, isoarborinol may represent the phylogenetic link between hopanols and sterols.
Arborane is a class of pentacyclic triterpene consisting of organic compounds with four 6-membered rings and one 5-membered ring. Arboranes are thought to be derived from arborinols, a class of natural cyclic triterpenoids typically produced by flowering plants. Thus arboranes are used as a biomarker for angiosperms and cordaites. Arborane is a stereoisomer of a compound called fernane, the diagenetic product of fernene and fernenol. Because aborinol and fernenol have different biological sources, the ratio of arborane/fernane in a sample can be used to reconstruct a record for the relative abundances of different plants.













