In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Brefeldin A is a lactone antiviral produced by the fungus Penicillium brefeldianum. Brefeldin A inhibits protein transport from the endoplasmic reticulum to the golgi complex indirectly by preventing association of COP-I coat to the Golgi membrane. Brefeldin A was initially isolated with hopes to become an antiviral drug but is now primarily used in research to study protein transport.

Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics such as the commonly prescribed erythromycin, azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and they are attached to the ring Desosamine which is crucial for bactericidal activity. The biological action of the desosamine-based macrolide antibiotics is to inhibit the bacterial ribosomal protein synthesis. These antibiotics which contain Desosamine are widely used to cure bacterial-causing infections in human respiratory system, skin, muscle tissues, and urethra.

N-Chlorosuccinimide ("NCS")is the organic compound with the formula C2H4(CO)2NCl. This white solid is used for chlorinations. It is also used as a mild oxidant. NCS is related to succinimide, but with N-Cl in place of N-H. The N–Cl bond is highly reactive, and NCS functions as a source of "Cl+".

Perimycin, also known as aminomycin and fungimycin, is polyene antibiotic produced by Streptomyces coelicolor var. aminophilus. The compound exhibits antifungal properties.
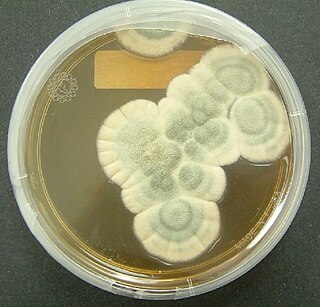
Penicillium chrysogenum is a species of fungus in the genus Penicillium. It is common in temperate and subtropical regions and can be found on salted food products, but it is mostly found in indoor environments, especially in damp or water-damaged buildings. It has been recognised as a species complex that includes P. notatum, P. meleagrinum, and P. cyaneofulvum. Molecular phylogeny has established that Alexander Fleming's first discovered penicillin producing strain is of a distinct species, P. rubens, and not of P. notatum. It has rarely been reported as a cause of human disease. It is the source of several β-lactam antibiotics, most significantly penicillin. Other secondary metabolites of P. chrysogenum include roquefortine C, meleagrin, chrysogine, 6-MSA YWA1/melanin, andrastatin A, fungisporin, secalonic acids, sorbicillin, and PR-toxin.

Lavendamycin is a naturally occurring chemical compound discovered in fermentation broth of the soil bacterium Streptomyces lavendulae. Lavendamycin has antibiotic properties and anti-proliferative effects against several cancer cell lines. The use of lavendamycin as a cytotoxic agent in cancer therapy failed due to poor water solubility and non-specific cytotoxicity. The study of lavendamycin-based analogs designed to overcome these liabilities has been an area of research.
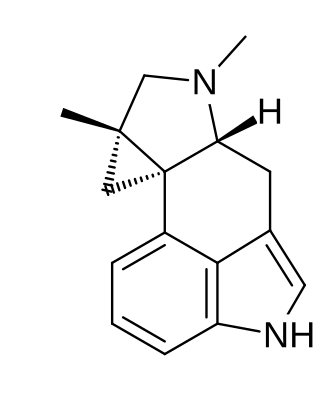
Cycloclavine is an ergot alkaloid. It was first isolated in 1969 from seeds of Ipomoea hildebrandtii vatke. The first total synthesis of (±)-cycloclavine was published in 2008 by Szántay. Further reports came from Wipf and Petronijevic, Cao and Brewer. In 2016, Wipf and McCabe completed an 8-step asymmetric synthesis of (–)-cycloclavine, and in 2018, they expanded this approach toward (+)-cycloclavine and a biological characterization of the binding profile of both enantiomers on 16 brain receptors. Natural (+)- and unnatural (–)-cycloclavine demonstrated significant stereospecificity and unique binding profiles in comparison to LSD, psilocin, and DMT. Differential 5-HT receptor affinities, as well as novel sigma-1 receptor properties, suggest potential future therapeutic opportunities of clavine alkaloid scaffolds.
Fungal isolates have been researched for decades. Because fungi often exist in thin mycelial monolayers, with no protective shell, immune system, and limited mobility, they have developed the ability to synthesize a variety of unusual compounds for survival. Researchers have discovered fungal isolates with anticancer, antimicrobial, immunomodulatory, and other bio-active properties. The first statins, β-Lactam antibiotics, as well as a few important antifungals, were discovered in fungi.
Medicinal fungi are fungi that contain metabolites or can be induced to produce metabolites through biotechnology to develop prescription drugs. Compounds successfully developed into drugs or under research include antibiotics, anti-cancer drugs, cholesterol and ergosterol synthesis inhibitors, psychotropic drugs, immunosuppressants and fungicides.

SCH-202,596 is a natural product which is a metabolite derived from an Aspergillus fungus. It acts as a selective non-peptide antagonist for the receptor GAL-1, which is usually activated by the neuropeptide galanin. SCH-202,596 is used for scientific research into this still little characterised receptor subtype.
Penicillium citrinum is an anamorph, mesophilic fungus species of the genus of Penicillium which produces tanzawaic acid A-D, ACC, Mevastatin, Quinocitrinine A, Quinocitrinine B, and nephrotoxic citrinin. Penicillium citrinum is often found on moldy citrus fruits and occasionally it occurs in tropical spices and cereals. This Penicillium species also causes mortality for the mosquito Culex quinquefasciatus. Because of its mesophilic character, Penicillium citrinum occurs worldwide. The first statin (Mevastatin) was 1970 isolated from this species.
Penicillium cyaneum is a species of the genus of Penicillium which was isolated from an oil-field. Penicillium cyaneum produces fatty acid, Brefeldin A and the antibiotic Cyanein
Penicillium paneum is a species of fungus in the genus Penicillium which can spoil cereal grains. Penicillium paneum produces 1-Octen-3-ol and penipanoid A, penipanoid B, penipanoid C, patulin and roquefortine C
Penicillium rubrum is a species of fungus in the genus Penicillium which produces kojic acid, mitorubrin, mitorubrinol, rubratoxin A, rubratoxin B rubralactone, rubramin and occurs in grain corn and soybeans. Penicillium rubrum is similar to the species Penicillium chrysogenum.
Penicillium thymicola is a halotolerant species of fungus in the genus Penicillium which produces okaramine A, daldinin D, alantrypinone, seranttrypinone, fumiquinazoline F and fumiquinazoline G.
Penicillium turbatum is an anamorph species of fungus in the genus Penicillium which was isolated from Taxus baccata. Penicillium turbatum produces pipolythiopiperazinedione-antibiotics, hyalodendrin A and hadacitin.
Streptomyces albidoflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil from Poland. Streptomyces albidoflavus produces dibutyl phthalate and streptothricins.
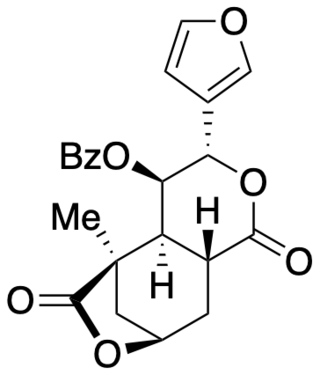
Collybolide is a secondary metabolite of the Rhodocollybia maculata mushroom, a basidiomycete fungus that grows on rotting conifer wood. It was previously believed to be a potent and selective kappa-opioid receptor agonist. However, a total synthesis and independent biological assay determined that collybolide neither excites nor suppresses kappa-opioid receptor signaling. Collybolide is unlikely to be psychoactive, although it has been shown to inhibit L-type calcium channels in isolated rat aorta.
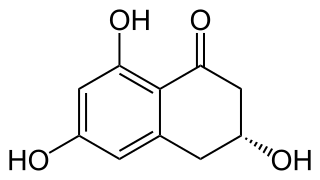
Scytalone or Scytolone is a cyclic beta hydroxy ketone substituted by hydroxy groups at positions 3, 6, and 8. It is a natural product found in various fungal species including Cytospora populina and Ceratocystis fimbriata.









