Erbium is a chemical element; it has symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, originally found in the gadolinite mine in Ytterby, Sweden, which is the source of the element's name.
Chlordane, or chlordan, is an organochlorine compound that was used as a pesticide. It is a white solid. In the United States, chlordane was used for termite-treatment of approximately 30 million homes until it was banned in 1988. Chlordane was banned 10 years earlier for food crops like corn and citrus, and on lawns and domestic gardens.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic compound with the formula C8H8O3. It is the methyl ester of salicylic acid. It is a colorless, viscous liquid with a sweet, fruity odor reminiscent of root beer (in which it is used as a flavoring), but often associatively called "minty", as it is an ingredient in mint candies. It is produced by many species of plants, particularly wintergreens. It is also produced synthetically, used as a fragrance and as a flavoring agent.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites, more commonly known as BPDE, react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.

Metabolomics is the scientific study of chemical processes involving metabolites, the small molecule substrates, intermediates, and products of cell metabolism. Specifically, metabolomics is the "systematic study of the unique chemical fingerprints that specific cellular processes leave behind", the study of their small-molecule metabolite profiles. The metabolome represents the complete set of metabolites in a biological cell, tissue, organ, or organism, which are the end products of cellular processes. Messenger RNA (mRNA), gene expression data, and proteomic analyses reveal the set of gene products being produced in the cell, data that represents one aspect of cellular function. Conversely, metabolic profiling can give an instantaneous snapshot of the physiology of that cell, and thus, metabolomics provides a direct "functional readout of the physiological state" of an organism. There are indeed quantifiable correlations between the metabolome and the other cellular ensembles, which can be used to predict metabolite abundances in biological samples from, for example mRNA abundances. One of the ultimate challenges of systems biology is to integrate metabolomics with all other -omics information to provide a better understanding of cellular biology.

The metabolome refers to the complete set of small-molecule chemicals found within a biological sample. The biological sample can be a cell, a cellular organelle, an organ, a tissue, a tissue extract, a biofluid or an entire organism. The small molecule chemicals found in a given metabolome may include both endogenous metabolites that are naturally produced by an organism as well as exogenous chemicals that are not naturally produced by an organism.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.

Orellanine or orellanin is a mycotoxin found in a group of mushrooms known as the Orellani within the family Cortinariaceae. Structurally, it is a bipyridine N-oxide compound somewhat related to the herbicide diquat.

Benzyl butyl phthalate (BBP) is an organic compound historically used a plasticizer, but which has now been largely phased out due to health concerns. It is a phthalate ester of containing benzyl alcohol, and n-butanol tail groups. Like most phthalates, BBP is non-volatile and remains liquid over a wide range of temperatures. It was mostly used as a plasticizer for PVC, but was also a common plasticizer for PVCA and PVB.

Caryophyllene, more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
Hexachlorobenzene, or perchlorobenzene, is an aryl chloride and a six-substituted chlorobenzene with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. It has been banned globally under the Stockholm Convention on Persistent Organic Pollutants.

Methedrone is a recreational drug of the cathinone chemical class. Chemically, methedrone is closely related to para-methoxymethamphetamine (PMMA), methylone and mephedrone. Methedrone received media attention in 2009 after the death of two young Swedish men. In both cases toxicology analysis showed methedrone was the only drug present in both men during the time of their overdose and subsequent deaths.

Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.

Pentachloronitrobenzene, typically abbreviated PCNB, is a registered fungicide formally derived from nitrobenzene. It is a off-white to yellow crystalline solid with a musty odor.
'MDMB-CHMICAa' is an indole-based synthetic cannabinoid that is a potent agonist of the CB1 receptor and has been sold online as a designer drug. While MDMB-CHMICA was initially sold under the name "MMB-CHMINACA", the compound corresponding to this code name (i.e. the isopropyl instead of t-butyl analogue of MDMB-CHMINACA) has been identified on the designer drug market in 2015 as AMB-CHMINACA.
Profenofos is an organophosphate insecticide. It is a liquid with a pale yellow to amber color and a garlic-like odor. It was first registered in the United States in 1982. As of 2015, it was not approved in the European Union.
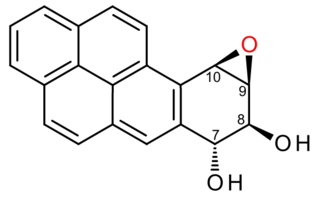
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.