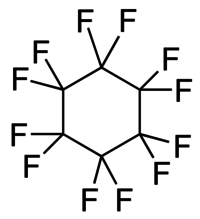
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene and is a PFAS that has numerous applications. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.
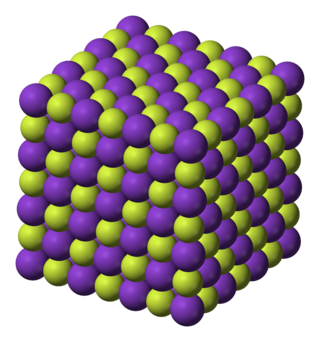
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.

Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.

Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour. The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol (F
3C)
2C(OH)
2, a geminal diol.

Carbon monofluoride (CF, CFx, or (CF)n), also called polycarbon monofluoride (PMF), polycarbon fluoride, poly(carbon monofluoride), and graphite fluoride, is a material formed by high-temperature reaction of fluorine gas with graphite, charcoal, or pyrolytic carbon powder. It is a highly hydrophobic microcrystalline powder. Its CAS number is 51311-17-2. In contrast to graphite intercalation compounds it is a covalent graphite compound.

Perfluorohexane, or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all of the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low-temperature applications due to its low boiling point of 56 °C and freezing point of −90 °C. It is odorless and colorless. Unlike typical hydrocarbons, the structure features a helical carbon backbone.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

FC-75 is a fluorocarbon derivative of tetrahydrofuran with the chemical formula C8F16O. It is practically insoluble in water.
Perfluorodecalin is a fluorocarbon, a derivative of decalin in which all of the hydrogen atoms are replaced by fluorine atoms. It is chemically and biologically inert and stable up to 400 °C. Several applications make use of its ability to dissolve gases.
The Fowler process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride.
Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.

Fluorographene (or perfluorographane, graphene fluoride) is a fluorocarbon derivative of graphene. It is a two dimensional carbon sheet of sp3 hybridized carbons, with each carbon atom bound to one fluorine. The chemical formula is (CF)n. In comparison, Teflon (polytetrafluoroethylene), -(CF2)n-, consists of carbon "chains" with each carbon bound to two fluorines.

Perfluoromethylcyclohexane is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon methylcyclohexane. It is chemically and biologically inert.

Henry Aaron Hill was an American chemist who became the first African American president of the American Chemical Society (ACS). As a scientist, he specialized in the chemistry of fluorocarbons.
Russell P. Hughes an American/British chemist, is the Frank R. Mori Professor Emeritus and Research Professor in the Department of Chemistry at Dartmouth College. His research interests are in organometallic chemistry, with emphasis on the chemistry of transition metal complexes interacting with fluorocarbons. His research group’s work in this area led to several creative syntheses of complexes of transition metal and perfluorinated hydrocarbon fragments.
Joseph H. Simons was a U.S. chemist who became famous for discovering one of the first practical ways to mass-produce fluorocarbons in the 1930s while a professor of chemical engineering at Pennsylvania State University. In 1950, he and other employees of 3M received a patent for the procedure of electrochemical fluorination.