
A carbonate is a salt of carbonic acid,, characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate groupO=C(−O−)2.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Sodium percarbonate or sodium carbonate peroxide is a chemical substance with empirical formula Na2H3CO6. It is an adduct of sodium carbonate and hydrogen peroxide whose formula is more properly written as 2 Na2CO3 · 3 H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.

Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity into two or more fragments. Chemical decomposition is usually regarded and defined as the exact opposite of chemical synthesis. In short, the chemical reaction in which two or more products are formed from a single reactant is called a decomposition reaction.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters.

The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. The iodine clock reaction exists in several variations, which each involve iodine species and redox reagents in the presence of starch. Two colourless solutions are mixed and at first there is no visible reaction. After a short time delay, the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide–starch complex. In some variations, the solution will repeatedly cycle from colorless to blue and back to colorless, until the reagents are depleted.
Sodium perborate are chemical compounds with chemical formula [Na+]2[B2O4(OH)4]2−(H2O)x. Commonly encountered salts are the anhydrous form (x = 0) and as a hexahydrate (x = 6). These two species are sometimes called, respectively, "monohydrate" or PBS-1 and "tetrahydrate" or PBS-4, after the historic assumption that NaBO3 would be the anhydrous form). Both the anhydrous and hexahydrate salts are white, odorless, water-soluble solids.
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.
The Wurtz–Fittig reaction is the chemical reaction of an aryl halide, alkyl halides, and sodium metal to give substituted aromatic compounds. Following the work of Charles Adolphe Wurtz on the sodium-induced coupling of alkyl halides, Wilhelm Rudolph Fittig extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.

Potassium bifluoride is the inorganic compound with the formula K[HF2]. This colourless salt consists of the potassium cation and the bifluoride anion. The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products.

Potassium peroxochromate, potassium tetraperoxochromate(V), or simply potassium perchromate, is an inorganic compound having the chemical formula K3[Cr(O2)4]. It is a red-brown paramagnetic solid. It is the potassium salt of tetraperoxochromate(V), one of the few examples of chromium in the +5 oxidation state and one of the rare examples of a complex stabilized only by peroxide ligands. This compound is used as a source of singlet oxygen.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.
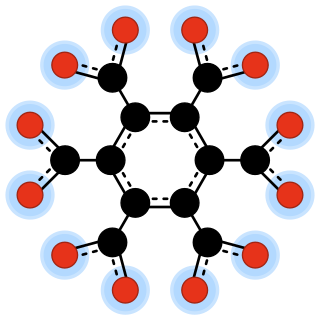
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula C
xOn−
y for some integers x, y, and n.

In chemistry, peroxydicarbonate is a divalent anion with the chemical formula C
2O2−
6. It is one of the oxocarbon anions, which consist solely of carbon and oxygen. Its molecular structure can be viewed as two carbonate anions joined so as to form a peroxide bridge –O–O–.

In chemistry, metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid. In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character.













