Condensation polymers are any kind of polymers formed through a condensation reaction—where molecules join together—losing small molecules as byproducts such as water or methanol. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of all degrees of polymerization, or by condensative chain polymerization, when the polymer is formed by sequential addition of monomers to an active site in a chain reaction. The main alternative forms of polymerization are chain polymerization and polyaddition, both of which give addition polymers.

Nylon is a generic designation for a family of synthetic polymers composed of polyamides. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many different property variations. Nylon polymers have found significant commercial applications in fabric and fibers, in shapes, and in films.
Aramid fibers are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated body armor fabric and ballistic composites, in marine cordage, marine hull reinforcement, and as an asbestos substitute. The name is a portmanteau of the words "aromatic" and "polyamide". F.T.C. gave the name in 1974.
A polyamide is a polymer with repeating units linked by amide bonds.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant toward hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.

Polyimide is a polymer of imide monomers belonging to the class of high performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.
Polyamide-imides are either thermosetting or thermoplastic, amorphous polymers that have exceptional mechanical, thermal and chemical resistant properties. Polyamide-imides are used extensively as wire coatings in making magnet wire. They are prepared from isocyanates and TMA in N-methyl-2-pyrrolidone (NMP). A prominent distributor of polyamide-imides is Solvay Specialty Polymers, which uses the trademark Torlon.

Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.

Nylon 6 or polycaprolactam is a polymer developed by Paul Schlack at IG Farben to reproduce the properties of nylon 6,6 without violating the patent on its production. It is a semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the comparison between condensation and addition polymers. Its competition with nylon 6,6 and the example it set have also shaped the economics of the synthetic fiber industry. It is sold under numerous trade names including Perlon (Germany), Dederon, Nylatron, Capron, Ultramid, Akulon, Kapron, and Durethan.

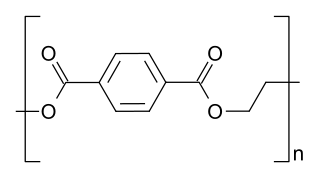
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term diamine refers mostly to primary diamines, as those are the most reactive.

4,4′-Oxydianiline is an organic compound with the formula O(C6H4NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless solid. This, the simplest diaryl ether, has a variety of niche applications.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the space shuttle.
Upilex is a heat-resistant polyimide film that is the product of the polycondensation reaction between biphenyl tetracarboxylic dianhydride (BPDA) monomers and a diamine. Its properties include dimensional stability, low water absorption, high chemical resistance and high mechanical properties, high heat and chemical resistance. It was developed by UBE Industries. Upilex-S is the standard grade but other grades include Upilex-RN, VT, CA and SGA. Upilex-S is used when excellent mechanical properties are required. Upilex-RN possesses excellent molding processability, while Upilex-VT has superior heat bonding characteristics. General applications of Upilex include their use in flexible printed circuits, electric motor and generator insulation, high temperature wire and cable wrapping, and specialty pressure sensitive tapes. Polyimides have also been extensively studied in gas and humidity sensors, where the concentration is determined by monitoring the capacitance of modified Upilex films. With advantages of flexibility and easy functionalization, Upilex films are often used as substrate materials in biosensor platforms. For instance, it is possible to electropolymerize onto these films or attach enzymes to it for the detection of glucose.

Bisphenol AF (BPAF) is a fluorinated organic compound that is an analogue of bisphenol A in which the two methyl groups are replaced with trifluoromethyl groups. It exists as a white to light-gray powder.

Polyfluorene is a polymer with formula (C
13H
8)
n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.

o-Cresolphthalein is a phthalein dye used as a pH indicator in titrations. It is insoluble in water but soluble in ethanol. Its solution is colourless below pH 8.2, and purple above 9.8. Its molecular formula is C22H18O4. It is used medically to determine calcium levels in the human body, or to synthesize polyamides or polyimides.

Cardo polymers are a sub group of polymers where carbons in the backbone of the polymer chain are also incorporated into ring structures. These backbone carbons are quaternary centers. As such, the cyclic side group lies perpendicular to the plane of the polymer chain, creating a looping structure. These rings are bulky structures which sterically hinder the polymers and prevent them from packing tightly. They also restrict the rotational range of motion of the polymer chain, creating a rigid backbone. As a result of their unique structures, these polymers have notably high thermal stability and solubility. There have been recent advances made in the applications of cardo polymers to membranes used for gas separation and transport.
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.














