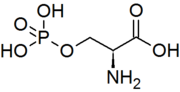A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.
A mitogen-activated protein kinase is a type of protein kinase that is specific to the amino acids serine and threonine. MAPKs are involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis.
Chemical biology is a scientific discipline between the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.
The Casein kinase 1 family of protein kinases are serine/threonine-selective enzymes that function as regulators of signal transduction pathways in most eukaryotic cell types. CK1 isoforms are involved in Wnt signaling, circadian rhythms, nucleo-cytoplasmic shuttling of transcription factors, DNA repair, and DNA transcription.
In molecular biology, biochemistry and cell signaling the kinome of an organism is the complete set of protein kinases encoded in its genome. Kinases are usually enzymes that catalyze phosphorylation reactions and fall into several groups and families, e.g., those that phosphorylate the amino acids serine and threonine, those that phosphorylate tyrosine and some that can phosphorylate both, such as the MAP2K and GSK families. The term was first used in 2002 by Gerard Manning and colleagues in twin papers analyzing the 518 human protein kinases, and refers to both protein kinases and protein pseudokinases and their evolution of protein kinases throughout the eukaryotes. Other kinomes have been determined for rice, several fungi, nematodes, and insects, sea urchins, Dictyostelium discoideum, and the process of infection by Mycobacterium tuberculosis. Although the primary sequence of protein kinases shows substantial divergence between unrelated eukaryotes, and amino acid differences in catalytic motifs have permitted their separation of kinomes into canonical and pseudokinase subtypes, the variation found in the amino acid motifs adjacent to the site of actual phosphorylation of substrates by eukaryotic kinases is much smaller.

CHEK2 is a tumor suppressor gene that encodes the protein CHK2, a serine-threonine kinase. CHK2 is involved in DNA repair, cell cycle arrest or apoptosis in response to DNA damage. Mutations to the CHEK2 gene have been linked to a wide range of cancers.

RAC(Rho family)-alpha serine/threonine-protein kinase is an enzyme that in humans is encoded by the AKT1 gene. This enzyme belongs to the AKT subfamily of serine/threonine kinases that contain SH2 protein domains. It is commonly referred to as PKB, or by both names as "Akt/PKB".
The IκB kinase is an enzyme complex that is involved in propagating the cellular response to inflammation, specifically the regulation of lymphocytes.

Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S. pombe, where it is encoded by genes cdc28 and cdc2, respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates ; phosphorylation of these proteins leads to cell cycle progression.
Mitogen-activated protein kinase 14, also called p38-α, is an enzyme that in humans is encoded by the MAPK14 gene.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Calcium/calmodulin-dependent protein kinase type II subunit alpha (CAMKIIα), a.k.a.Ca2+/calmodulin-dependent protein kinase II alpha, is one subunit of CamKII, a protein kinase (i.e., an enzyme which phosphorylates proteins) that in humans is encoded by the CAMK2A gene.

Ribosomal protein S6 kinase alpha-1 is an enzyme that in humans is encoded by the RPS6KA1 gene.

Mitogen-activated protein kinase kinase kinase 1 (MAP3K1) is a signal transduction enzyme that in humans is encoded by the autosomal MAP3K1 gene.

TBK1 is an enzyme with kinase activity. Specifically, it is a serine / threonine protein kinase. It is encoded by the TBK1 gene in humans. This kinase is mainly known for its role in innate immunity antiviral response. However, TBK1 also regulates cell proliferation, apoptosis, autophagy, and anti-tumor immunity. Insufficient regulation of TBK1 activity leads to autoimmune, neurodegenerative diseases or tumorigenesis.

Ribosomal protein S6 kinase alpha-2 is an enzyme that in humans is encoded by the RPS6KA2 gene.

Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become either activated or deactivated, or otherwise modifying its function. Approximately 13000 human proteins have sites that are phosphorylated.

In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans. It is implicated in the development and progression of melanomas.

Wee1 is a nuclear kinase belonging to the Ser/Thr family of protein kinases in the fission yeast Schizosaccharomyces pombe. Wee1 has a molecular mass of 96 kDa and is a key regulator of cell cycle progression. It influences cell size by inhibiting the entry into mitosis, through inhibiting Cdk1. Wee1 has homologues in many other organisms, including mammals.