
Mentha, also known as mint, is a genus of flowering plants in the mint family, Lamiaceae. It is estimated that 13 to 24 species exist, but the exact distinction between species is unclear. Hybridization occurs naturally where some species' ranges overlap. Many hybrids and cultivars are known.

Peppermint is a hybrid species of mint, a cross between watermint and spearmint. Indigenous to Europe and the Middle East, the plant is now widely spread and cultivated in many regions of the world. It is occasionally found in the wild with its parent species.

Spearmint, also known as garden mint, common mint, lamb mint and mackerel mint, is native to Europe and southern temperate Asia, extending from Ireland in the west to southern China in the east. It is naturalized in many other temperate parts of the world, including northern and southern Africa, North America, and South America. It is used as a flavouring in food and herbal teas. The aromatic oil, called oil of spearmint, is also used as a flavoring and sometimes as a scent.

An essential oil is a concentrated hydrophobic liquid containing volatile chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does not mean required or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism.

Menthol is an organic compound, specifically a monoterpenoid, that occurs naturally in the oils of several plants in the mint family, such as corn mint and peppermint. It is a white or clear waxy crystalline substance that is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration.
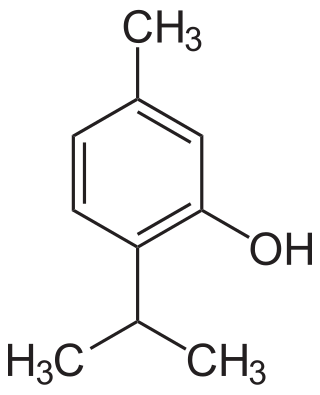
Thymol, C10H14O, is a natural monoterpenoid phenol derivative of p-Cymene, isomeric with carvacrol. It occurs naturally in the oil of thyme, and it is extracted from Thymus vulgaris, ajwain, and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris. Thymol is only slightly soluble in water at neutral pH, but it is extremely soluble in alcohols and other organic solvents. It is also soluble in strongly alkaline aqueous solutions due to deprotonation of the phenol. Its dissociation constant (pKa) is 10.59±0.10. Thymol absorbs maximum UV radiation at 274 nm.

Monarda is a genus of flowering plants in the mint family, Lamiaceae. The genus is endemic to North America. Common names include bergamot, bee balm, horsemint, and oswego tea, the first being inspired by the fragrance of the leaves, which is reminiscent of bergamot orange. The genus was named for the Spanish botanist Nicolás Monardes, who wrote a book in 1574 describing plants of the New World.

Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway, spearmint, and dill.

Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal. It is classified as a monoterpenoid, which means that it is an oxidized derivative of a terpene, a large class of naturally occurring C10 hydrocarbons.
Menthone is a chemical compound of the monoterpene class of naturally occurring organic compounds found in a number of essential oils, one that presents with minty flavor. It is a specific pair of stereoisomers of the four possible such isomers for the chemical structure, 2-isopropyl-5-methylcyclohexanone. Of those, the stereoisoomer l-menthone—formally, the (2S,5R)-trans isomer of that structure, as shown at right—is the most abundant in nature. Menthone is structurally related to menthol, which has a secondary alcohol (>C-OH) in place of the carbon-oxygen double bond projecting from the cyclohexane ring.
Phellandrenes are organic compounds with the formula C10H20. have a similar molecular structure and similar chemical properties. α-Phellandrene and β-phellandrene are cyclic monoterpenes and are double-bond isomers. In α-phellandrene, both double bonds are endocyclic, and in β-phellandrene, one of them is exocyclic. Both are insoluble in water, but miscible with organic solvents.

Mentha arvensis, the corn mint, field mint, or wild mint, is a species of flowering plant in the mint family Lamiaceae. It has a circumboreal distribution, being native to the temperate regions of Europe and western and central Asia, east to the Himalaya and eastern Siberia, and North America. Mentha canadensis, the related species, is also included in Mentha arvensis by some authors as two varieties, M. arvensis var. glabrata Fernald and M. arvensis var. piperascens Malinv. ex L. H. Bailey.

Eucalyptus oil is the generic name for distilled oil from the leaves of Eucalyptus, a genus of the plant family Myrtaceae, mostly native to Australia but cultivated worldwide. Eucalyptus oil has a history of wide application, as a pharmaceutical, antiseptic, repellent, flavouring and fragrance, as well as having industrial uses. The leaves of selected Eucalyptus species are steam distilled to extract eucalyptus oil.
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries.

Eucalyptus dives, commonly known as the broad-leaved peppermint or blue peppermint, is a species of tree that is endemic to south-eastern Australia. It has rough, finely fibrous bark on the trunk and larger branches, smooth bark above, lance-shaped or curved adult leaves, flower buds in groups of eleven or more, white flowers and cup-shaped, hemispherical or conical fruit.

Eucalyptus piperita, commonly known as Sydney peppermint and urn-fruited peppermint, is a small to medium forest tree native to New South Wales, Australia.

Mentha canadensis is a species of mint native to North America and the eastern part of Asia. In North America, it is commonly known as Canada mint, American wild mint, and in Asia as Chinese mint, Sakhalin mint, Japanese mint, and East Asian wild mint. The flowers are bluish or have a slight violet tint. The plant is upright, growing to about 4–18 in (10–46 cm) tall. Leaves grow opposite from each other, and flower bunches appear in the upper leaf axils. The mint grows in wet areas but not directly in water, so it will be found near sloughs, and lake and river edges. Plants bloom from July to August in their native habitats.

Aromatherapy is a practice based on the use of aromatic materials, including essential oils and other aroma compounds, with claims for improving psychological well-being. It is used as a complementary therapy or as a form of alternative medicine, and typically is used via inhalation and not by ingestion.
Mentha grandiflora is a plant species in the genus Mentha, endemic to eastern Australia. The species was described in 1848 by botanist George Bentham. Its epithet, grandiflora, means "with large flowers".

Pinocarvone is a terpenoid. Structurally, it is a bicyclic ketone.

















