
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to the cytoplasm of eukaryotic cells, some bacteria and some archaea. A microtubule can grow as long as 50 micrometres and are highly dynamic. The outer diameter of a microtubule is about 24 nm while the inner diameter is about 12 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound. Deacetylation is the removal of an acetyl group.
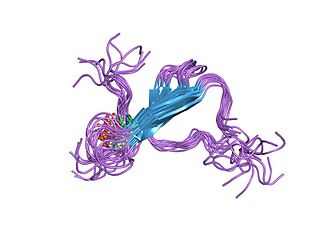
Tau proteins are proteins that stabilize microtubules. They are abundant in neurons of the central nervous system and are less common elsewhere, but are also expressed at very low levels in CNS astrocytes and oligodendrocytes. Pathologies and dementias of the nervous system such as Alzheimer's disease and Parkinson's disease are associated with tau proteins that have become defective and no longer stabilize microtubules properly.
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton.

A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. Humans, animals, and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation.
Polyglycylation is a form of posttranslational modification of glutamate residues of the carboxyl-terminal region tubulin in certain microtubules originally discovered in Paramecium, and later shown in mammalian neurons as well.
In cell biology, microtubule nucleation is the event that initiates de novo formation of microtubules (MTs). These filaments of the cytoskeleton typically form through polymerization of α- and β-tubulin dimers, the basic building blocks of the microtubule, which initially interact to nucleate a seed from which the filament elongates.

Microtubule-associated protein 2 is a protein that in humans is encoded by the MAP2 gene.

Microtubule-associated protein 4 is a protein that in humans is encoded by the MAP4 gene.
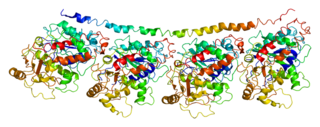
Tubulin alpha-4A chain is a protein that in humans is encoded by the TUBA4A gene.
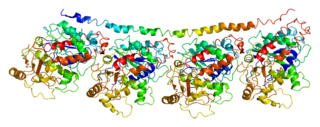
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.

Tubulin alpha-1B chain is a protein that in humans is encoded by the TUBA1B gene.

Microtubule-associated protein 1A is a protein that in humans is encoded by the MAP1A gene.
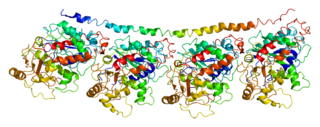
Tubulin alpha-3C/D chain is a protein that in humans is encoded by the TUBA3C gene.

Tubulin beta chain is a protein that in humans is encoded by the TUBB gene.
The Walker A and Walker B motifs are protein sequence motifs, known to have highly conserved three-dimensional structures. These were first reported in ATP-binding proteins by Walker and co-workers in 1982.
Tubulin inhibitors are drugs that interfere directly with the tubulin system, which is in contrast to those drugs acting on DNA for cancer chemotherapy. Microtubules play an important role in eukaryotic cells. Alpha- and beta-tubulin, the main components of microtubules, have gained considerable interest because of their function and biophysical properties and has become the subject of intense study. The addition of tubulin ligands can affect microtubule stability and function, including mitosis, cell motion and intracellular organelle transport. Tubulin binding molecules have generated significant interest after the introduction of the taxanes into clinical oncology and the general use of the vinca alkaloids. These compounds inhibit cell mitosis by binding to the protein tubulin in the mitotic spindle and preventing polymerization or depolymerization into the microtubules. This mode of action is also shared with another natural agent called colchicine.

Tubulin, gamma complex associated protein 4 is a protein in humans that is encoded by the TUBGCP4 gene. It is part of the gamma tubulin complex, which required for microtubule nucleation at the centrosome.
Detyrosination is a form of posttranslational modification that occurs on alpha-tubulin. It consists of the removal of the C-terminal tyrosine to expose a glutamate at the newly formed C-terminus. Tubulin polymers, called microtubules, that contain detyrosinated alpha-tubulin are usually referred to as Glu-microtubules while unmodified polymers are called Tyr-microtubules.












