Related Research Articles

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can grow as long as 50 micrometres and are highly dynamic. The outer diameter of a microtubule is between 23 and 27 nm while the inner diameter is between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.
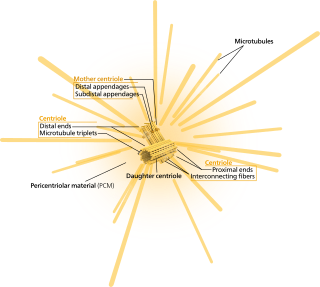
In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use structures other than MTOCs to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.

Post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

Dynein is a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport, thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus end.
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound. Deacetylation is the removal of an acetyl group.

Motor proteins are a class of molecular motors that can move along the cytoplasm of animal cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.

Stathmin, also known as metablastin and oncoprotein 18 is a protein that in humans is encoded by the STMN1 gene.
Polyglutamylation is a form of reversible posttranslational modification of glutamate residues seen for example in alpha and beta tubulins, nucleosome assembly proteins NAP1 and NAP2. The γ-carboxy group of glutamate may form peptide-like bond with the amino group of a free glutamate whose α-carboxy group can now be extended into a polyglutamate chain. The glutamylation is done by the enzyme glutamylase and removed by deglutamylase.
In cell biology, microtubule nucleation is the event that initiates de novo formation of microtubules (MTs). These filaments of the cytoskeleton typically form through polymerization of α- and β-tubulin dimers, the basic building blocks of the microtubule, which initially interact to nucleate a seed from which the filament elongates.

Class III β-tubulin, otherwise known as βIII-tubulin (β3-tubulin) or β-tubulin III, is a microtubule element of the tubulin family found almost exclusively in neurons, and in testis cells. In humans, it is encoded by the TUBB3 gene.
In enzymology, an alpha-tubulin N-acetyltransferase is an enzyme which is encoded by the ATAT1 gene.
Tubulin alpha-4A chain is a protein that in humans is encoded by the TUBA4A gene.
Tektins are cytoskeletal proteins found in cilia and flagella as structural components of outer doublet microtubules. They are also present in centrioles and basal bodies. They are polymeric in nature, and form filaments.
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.
Tubulin alpha-3C/D chain is a protein that in humans is encoded by the TUBA3C gene.
Pericentriolar material is an amorphous mass of protein which makes up the part of the animal centrosome that surrounds the two centrioles. The PCM contains proteins responsible for microtubule nucleation and anchoring including γ-tubulin, pericentrin and ninein.
Tubulin inhibitors are chemotherapy drugs that interfere directly with the tubulin system, which is in contrast to those chemotherapy drugs acting on DNA. Microtubules play an important role in eukaryotic cells. Alpha- and beta-tubulin, the main components of microtubules, have gained considerable interest because of their function and biophysical properties and has become the subject of intense study. The addition of tubulin ligands can affect microtubule stability and function, including mitosis, cell motion and intracellular organelle transport. Tubulin binding molecules have generated significant interest after the introduction of the taxanes into clinical oncology and the general use of the vinca alkaloids. These compounds inhibit cell mitosis by binding to the protein tubulin in the mitotic spindle and preventing polymerization or depolymerization into the microtubules. This mode of action is also shared with another natural agent called colchicine.
Detyrosination is a form of posttranslational modification that occurs on alpha-tubulin. It consists of the removal of the C-terminal tyrosine to expose a glutamate at the newly formed C-terminus. Tubulin polymers, called microtubules, that contain detyrosinated alpha-tubulin are usually referred to as Glu-microtubules while unmodified polymers are called Tyr-microtubules.
H3K27ac is an epigenetic modification to the DNA packaging protein Histone H3. It is a mark that indicates the acetylation at the 27th lysine residue of the histone H3 protein.
References
- ↑ Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bré MH (1994). "Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules". Science. 266 (5191): 1688–1691. doi:10.1126/science.7992051. PMID 7992051.
- ↑ Banerjee Asok (2002). "Coordination of posttranslational modifications of bovine brain alpha-tubulin. Polyglycylation of delta2 tubulin". J. Biol. Chem. 277 (48): 46140–46144. doi: 10.1074/jbc.M208065200 . PMID 12356754.