An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids.

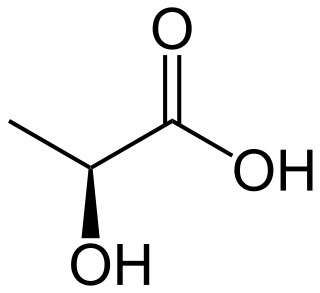
Lactic acid is an organic acid. It has the molecular formula CH3CH(OH)COOH. It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate. The name of the derived acyl group is lactoyl.

Salami is a cured sausage consisting of fermented and air-dried meat, typically pork. Historically, salami was popular among Southern, Eastern, and Central European peasants because it can be stored at room temperature for up to 45 days once cut, supplementing a potentially meager or inconsistent supply of fresh meat. Countries and regions across Europe make their own traditional varieties of salami.
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.

Lactic acidosis is a medical condition characterized by a build-up of lactate in the body, with formation of an excessively low pH in the bloodstream. It is a form of metabolic acidosis, in which excessive acid accumulates due to a problem with the body's oxidative metabolism.
Sodium stearoyl-2-lactylate is a versatile, FDA approved food additive used to improve the mix tolerance and volume of processed foods. It is one type of a commercially available lactylate. SSL is non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Because SSL is a safe and highly effective food additive, it is used in a wide variety of products ranging from baked goods and desserts to pet foods.

Calcium lactate is a white crystalline salt with formula C
6H
10CaO
6, consisting of two lactate anions H
3C(CHOH)CO−
2 for each calcium cation Ca2+
. It forms several hydrates, the most common being the pentahydrate C
6H
10CaO
6·5H
2O.
Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.
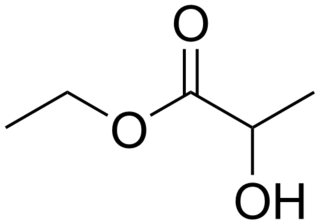
Ethyl lactate, also known as lactic acid ethyl ester, is the organic compound with the formula CH3CH(OH)CO2CH2CH3. It is the ethyl ester of lactic acid. A colorless liquid, it is a chiral ester. Being naturally derived, it is readily available as a single enantiomer. It is commonly used as a solvent. This compound is considered biodegradable and can be used as a water-rinsible degreaser. Ethyl lactate is found naturally in small quantities in a wide variety of foods including wine, chicken, and various fruits. The odor of ethyl lactate when dilute is mild, buttery, creamy, with hints of fruit and coconut.

Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature.

Fermentation is a metabolic process that produces chemical changes in organic substances through the action of enzymes. In biochemistry, it is broadly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.

Disodium pyrophosphate or sodium acid pyrophosphate (SAPP) is an inorganic compound consisting of sodium cations and pyrophosphate anion. It is a white, water-soluble solid that serves as a buffering and chelating agent, with many applications in the food industry. When crystallized from water, it forms a hexahydrate, but it dehydrates above room temperature. Pyrophosphate is a polyvalent anion with a high affinity for polyvalent cations, e.g. Ca2+.

Sodium lactate is the sodium salt of lactic acid, and has a mild saline taste. It is produced by fermentation of a sugar source, such as corn or beets, and then, by neutralizing the resulting lactic acid to create a compound having the formula NaC3H5O3.

A fire extinguisher is a handheld active fire protection device usually filled with a dry or wet chemical used to extinguish or control small fires, often in emergencies. It is not intended for use on an out-of-control fire, such as one which has reached the ceiling, endangers the user, or otherwise requires the equipment, personnel, resources or expertise of a fire brigade. Typically, a fire extinguisher consists of a hand-held cylindrical pressure vessel containing an agent that can be discharged to extinguish a fire. Fire extinguishers manufactured with non-cylindrical pressure vessels also exist but are less common.
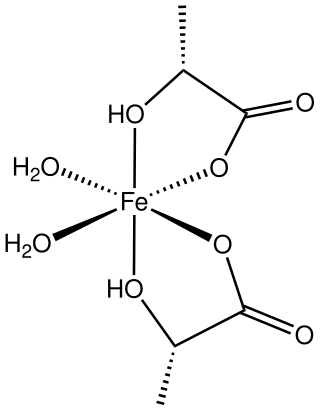
Ferrous lactate, or iron(II) lactate, are coordination complexes of iron(II) with one or more lactate ligands. One example is Fe(lactate)2(H2O)2(H2O) where lactate is CH3CH(OH)CO−2. It is a colorless solid.

Biopreservation is the use of natural or controlled microbiota or antimicrobials as a way of preserving food and extending its shelf life. The biopreservation of food, especially utilizing lactic acid bacteria (LAB) that are inhibitory to food spoilage microbes, has been practiced since early ages, at first unconsciously but eventually with an increasingly robust scientific foundation. Beneficial bacteria or the fermentation products produced by these bacteria are used in biopreservation to control spoilage and render pathogens inactive in food. There are a various modes of action through which microorganisms can interfere with the growth of others such as organic acid production, resulting in a reduction of pH and the antimicrobial activity of the un-dissociated acid molecules, a wide variety of small inhibitory molecules including hydrogen peroxide, etc. It is a benign ecological approach which is gaining increasing attention.
Lactylates are organic compounds that are FDA approved for use as food additives and cosmetic ingredients, e.g. as food-grade emulsifiers. These additives are non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Owing to their safety and versatile functionality, lactylates are used in a wide variety of food and non-food applications. In the United States, the Food Chemicals Codex specifies the labeling requirements for food ingredients including lactylates. In the European Union, lactylates must be labelled in accordance with the requirements of the applicable EU regulation. Lactylates may be labelled as calcium stearoyl lactylate (CSL), sodium stearoyl lactylate (SSL), or lactylic esters of fatty acids (LEFA).













