
Germanium is a chemical element; it has symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.

Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Curium(III) oxide is a compound composed of curium and oxygen with the chemical formula Cm2O3. It is a crystalline solid with a unit cell that contains two curium atoms and three oxygen atoms. The simplest synthesis equation involves the reaction of curium(III) metal with O2−: 2 Cm3+ + 3 O2− ---> Cm2O3. Curium trioxide can exist as five polymorphic forms. Two of the forms exist at extremely high temperatures, making it difficult for experimental studies to be done on the formation of their structures. The three other possible forms which curium sesquioxide can take are the body-centered cubic form, the monoclinic form, and the hexagonal form. Curium(III) oxide is either white or light tan in color and, while insoluble in water, is soluble in inorganic and mineral acids. Its synthesis was first recognized in 1955.

Titanocene dichloride is the organotitanium compound with the formula (η5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug.
Organogermanium chemistry is the science of chemical species containing one or more C–Ge bonds. Germanium shares group 14 in the periodic table with carbon, silicon, tin and lead. Historically, organogermanes are considered as nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with their organosilicon and organoboron analogues in some cross-coupling reactions.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives. Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.
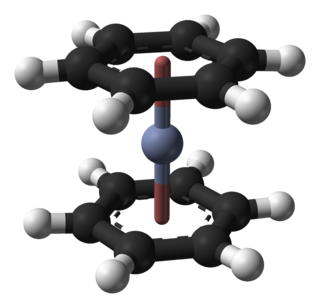
Bis(benzene)chromium is the organometallic compound with the formula Cr(η6-C6H6)2. It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical complex containing two arene ligands.

Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.

Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organometallic group 2 compounds are rare and are typically limited to academic interests.

Germanium dichloride is a chemical compound of germanium and chlorine with the formula GeCl2. It is a yellow solid. Germanium dichloride is an example of a compound featuring germanium in the +2 oxidation state.

Germylenes are a class of germanium(II) compounds with the general formula :GeR2. They are heavier carbene analogs. However, unlike carbenes, whose ground state can be either singlet or triplet depending on the substituents, germylenes have exclusively a singlet ground state. Unprotected carbene analogs, including germylenes, has a dimerization nature. Free germylenes can be isolated under the stabilization of steric hindrance or electron donation. The synthesis of first stable free dialkyl germylene was reported by Jutzi, et al in 1991.

Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.
Germanium(II) hydroxide, normally written as Ge(OH)2, is a poorly characterised compound, sometimes called hydrous germanium(II) oxide or germanous hydroxide. It was first reported by Winkler in 1886.

Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2-, which is C
8H2−
8) bound to an actinide-metal center (An) in the oxidation state IV, resulting in the general formula An(C8H8)2.


















