
The pomegranate is a fruit-bearing deciduous shrub in the family Lythraceae, subfamily Punicoideae, that grows between 5 and 10 m tall.

Punicalagin is an ellagitannin, a type of phenolic compound. It is found in forms alpha and beta in pomegranates, in Terminalia catappa and Terminalia myriocarpa, and in Combretum molle, the velvet bushwillow, a plant species found in South Africa. These three genera are all Myrtales and the last two are both Combretaceae.
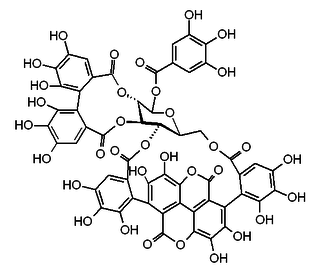
1-α-O-Galloylpunicalagin is an ester of gallic acid and punicalagin, a type of ellagitannin. It is found in the pomegranate and in Combretum glutinosum.
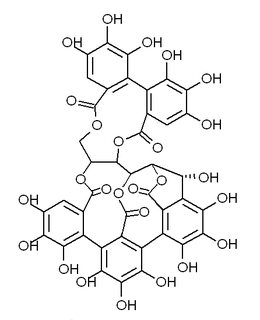
Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.

Corilagin is an ellagitannin. Corilagin was first isolated in 1951 from Dividivi extract and from Caesalpinia coriaria, hence the name of the molecule. It can also be found in Alchornea glandulosa and in the leaves of Punica granatum (pomegranate).
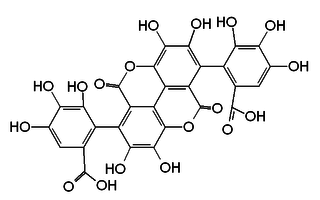
Gallagic acid is a polyphenolic chemical compound that can be found in the ellagitannins, a type of tannin, found in Punica granatum (pomegranate). It is a building block of the corresponding tannin punicalagin, punicalin, punicacortein C and 2-O-galloyl-punicalin.

1,2,3,4,6-Pentagalloylglucose is the pentagallic acid ester of glucose. It is a gallotannin and the precursor of ellagitannins.

Punigluconin is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Emblica officinalis. It is a molecule having a hexahydroxydiphenic acid group and two gallic acids attached to a gluconic acid core.

Punicacortein A is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Osbeckia chinensis.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.
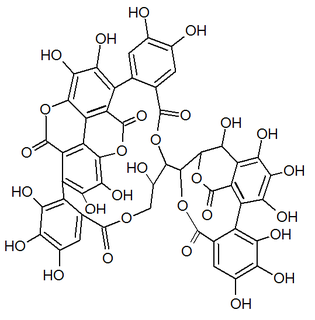
Punicacortein C is an ellagitannin, a phenolic compound. It is found in the bark of Punica granatum (pomegranate). The molecule contains a gallagic acid component.

Punicacortein B is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate).

Punicafolin is an ellagitannin from the leaves of Punica granatum (pomegranate) and in Phyllanthus emblica.

Granatin A is an ellagitannin found in the pericarp of Punica granatum (pomegranate). It is a weak carbonic anhydrase inhibitor.

Granatin B is an ellagitannin found in the fruit of Punica granatum (pomegranate). It is a molecule having an enantiomeric dehydrohexahydroxydiphenoyl group.

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Combretum molle, the velvet bushwillow, is a plant species in the genus Combretum found in West-, East- and South Africa.

Casuarinin is an ellagitannin. It is found in the pericarp of pomegranates. It is also found in Casuarina and Stachyurus species and in Alnus sieboldiana.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.
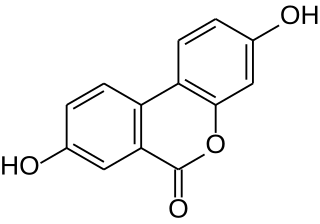
Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, and walnuts. Since the 2000s, urolithin A has been subject of preliminary studies regarding its possible biological effects.




















