
Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".
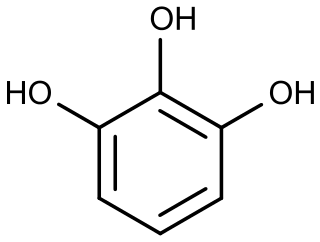
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a water-soluble, white solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomers of benzenetriols.
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.

Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid.
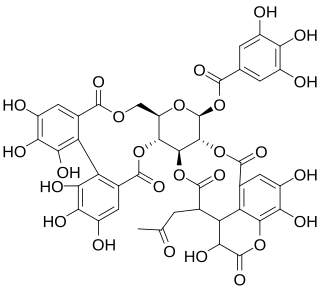
Chebulagic acid is a benzopyran tannin and an antioxidant that has many potential uses in medicine.

Ethyl gallate is a food additive with E number E313. It is the ethyl ester of gallic acid. Ethyl gallate is added to food as an antioxidant.
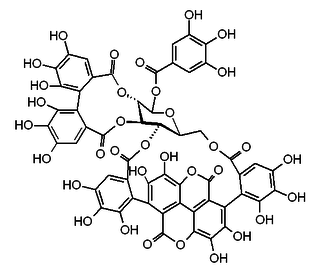
1-α-O-Galloylpunicalagin is an ester of gallic acid and punicalagin, a type of ellagitannin. It is found in the pomegranate and in Combretum glutinosum.

The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Casuarictin is an ellagitannin, a type of hydrolysable tannin. It can be found in Casuarina and Stachyurus species.

Punigluconin is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Emblica officinalis. It is a molecule having a hexahydroxydiphenic acid group and two gallic acids attached to a gluconic acid core.

Lambertianin C is an ellagitannin.

Sanguiin H-6 is an ellagitannin.
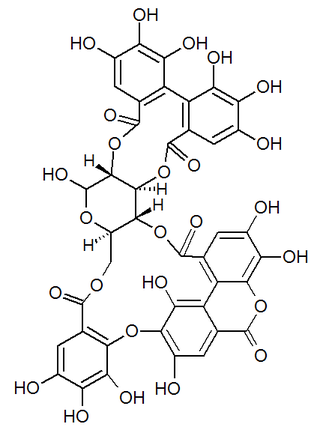
Alnusiin is an ellagitannin found in Alnus sieboldiana.

Geraniin is a dehydroellagitannin found in geraniums. It is found for instance in Geranium thunbergii, which is one of the most popular folk medicines and also an official antidiarrheic drug in Japan. It can also be found in the rind of Nephelium lappaceum (rambutan).

Sanguisorbic acid is a constituent of some ellagitannins. It is constituted by a hexahydroxydiphenic acid unit linked by an O-C bond to a gallic acid. The differences with its isomers, valoneic acid and nonahydroxytriphenic acid, are that the hydroxyl that links the hexahydroxydiphenoyl (HHDP) group to the galloyl group belongs to the galloyl group in valoneic acid, while in nonahydroxytriphenic acid, the hexahydroxydiphenic acid unit is linked by a C-C bond to gallic acid.

Nupharin A is an ellagitannin found in Nuphar japonica. It is a molecule with three gallic acid units and one hexahydroxydiphenic acid unit attached to a glucose residue. It is an isomer of punicafolin and tellimagrandin II.

Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.
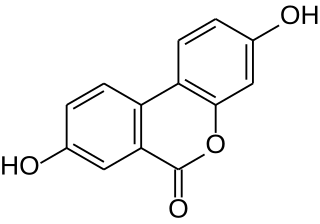
Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, walnuts, and others.
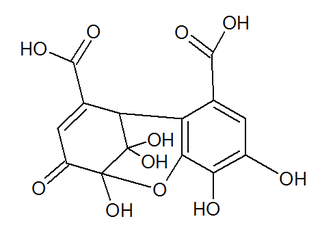
Dehydrohexahydroxydiphenic acid is a group found in dehydroellagitannins. It is formed from hexahydroxydiphenic acid (HHDP) through oxidation of the plant hydrolysable tannins. It is found in ellagitannins such as euphorbin A, geraniin or mallotusinic acid.

Mallojaponin is a hydrolysable tannin found in the bark of Mallotus japonicus. This compound contains the moiety elaeocarpusinic acid, an oxidized hexahydroxydiphenic acid group which reacted with a dehydroascorbic acid molecule. It also contains a valoneic acid and a gallic acid moieties linked to a glucose molecule.




















