| |
| Names | |
|---|---|
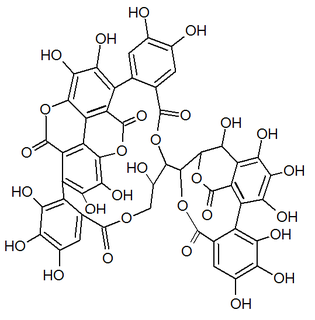
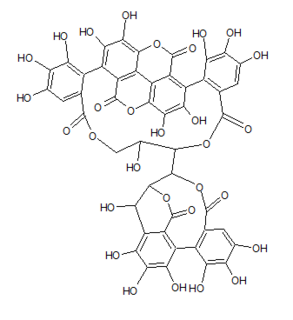
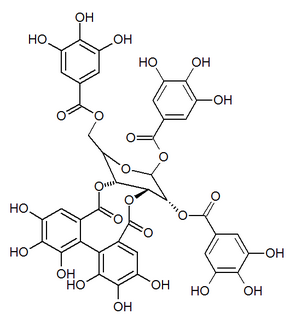
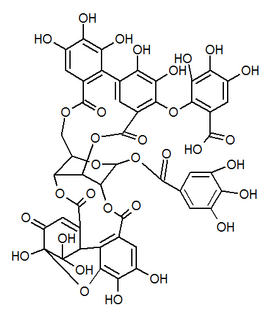
| IUPAC name 3,4,5-Trihydroxy-2-({(1'R,3R,3aS,4'R,5'S,6S,6aR,23'R,25'S,26'R,35'R,36'R,37'R)-3a,6,11',12',15',16',17',31',32',36',37'-undecahydroxy-2,2',7',20',28',41'-hexaoxo-25'-[(3,4,5-trihydroxybenzoyl)oxy]-3a, 5,6,6a-tetrahydrospiro[furo[3,2-b]furan-3,39'-[3,6,21,24,27,38,42]heptaoxanonacyclo[35.2.2.133,36.01,35.04,23.05,26.08,13.014,19.029,34]dotetraconta[8,10,12,14,16,18,29,31,33]nonaen]-10' -yl}oxy)benzoic acid | |
| Other names 1-O-Galloyl-2,4-elaeocarpusinoyl-3,6-(R)-valoneayl-beta-D-glucose | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| |
| |
| Properties | |
| C54H38O37 | |
| Molar mass | 1278.86 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mallojaponin is a hydrolysable tannin found in the bark of Mallotus japonicus . [1] This compound contains the moiety elaeocarpusinic acid, an oxidized hexahydroxydiphenic acid group (dehydrohexahydroxydiphenic acid or DHHDP) which reacted with a dehydroascorbic acid molecule. It also contains a valoneic acid and a gallic acid moieties linked to a glucose molecule.