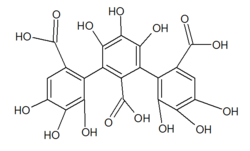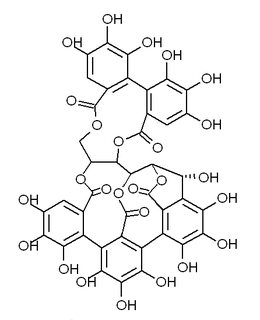
Castalagin is an ellagitannin, a type of hydrolyzable tannin, found in oak and chestnut wood and in the stem barks of Anogeissus leiocarpus and Terminalia avicennoides.
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds.

Grandinin is an ellagitannin. It can be found in Melaleuca quinquenervia leaves and in oaks species like the North American white oak and European red oak. It shows antioxydant activity. It is an astringent compound. It is also found in wine, red or white, aged in oak barrels.
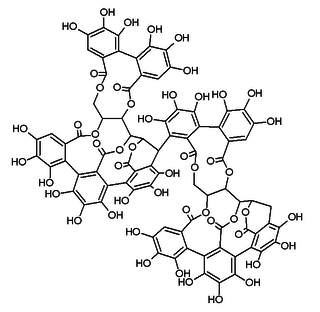
Roburin A is a tannin found in oak wood or oak cork.
The Flavono-ellagitannins or complex tannins are a class of tannins formed from the complexation of an ellagitannin with a flavonoid. Flavono-ellagitannins can be found in Quercus mongolica var. grosseserrata.

Punigluconin is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Emblica officinalis. It is a molecule having a hexahydroxydiphenic acid group and two gallic acids attached to a gluconic acid core.

Punicacortein A is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate) and in Osbeckia chinensis.

Punicalin is an ellagitannin. It can be found in Punica granatum (pomegranate) or in the leaves of Terminalia catappa, a plant used to treat dermatitis and hepatitis. It is also reported in Combretum glutinosum, all three species being Myrtales, the two last being Combretaceae.
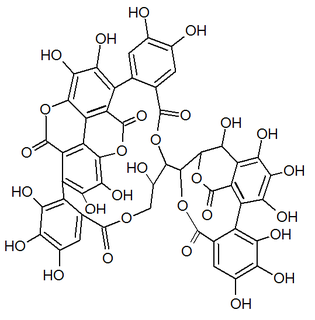
Punicacortein C is an ellagitannin, a phenolic compound. It is found in the bark of Punica granatum (pomegranate). The molecule contains a gallagic acid component.
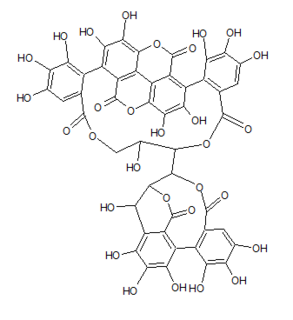
Punicacortein D is an ellagitannin, a type of phenolic compound. It is found in the bark and heartwood of Punica granatum (pomegranate). The molecule contains a gallagic acid component.

Punicacortein B is an ellagitannin, a polyphenol compound. It is found in the bark of Punica granatum (pomegranate).

The pomegranate ellagitannins, which include punicalagin isomers, are ellagitannins found in the sarcotestas, rind (peel), bark or heartwood of pomegranates.

Pedunculagin is an ellagitannin. It is formed from casuarictin via the loss of a gallate group.

Lambertianin C is an ellagitannin.

Sanguiin H-6 is an ellagitannin.

Lambertianin D is an ellagitannin found in Rubus lambertianus.
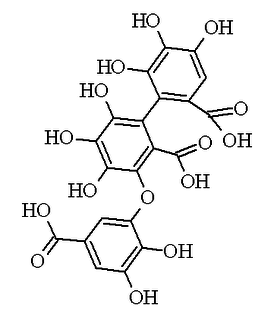
Sanguisorbic acid is a constituent of some ellagitannins. It is constituted by a hexahydroxydiphenic acid unit linked by an O-C bond to a gallic acid. The differences with its isomers, valoneic acid and nonahydroxytriphenic acid, are that the hydroxyl that links the hexahydroxydiphenoyl (HHDP) group to the galloyl group belongs to the galloyl group in valoneic acid, while in nonahydroxytriphenic acid, the hexahydroxydiphenic acid unit is linked by a C-C bond to gallic acid.
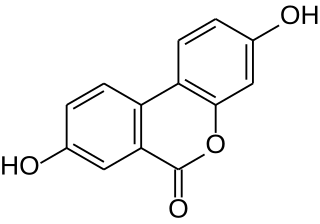
Urolithins are microflora human metabolites of dietary ellagic acid derivatives such as ellagitannins. They are produced in the human gut, and found in the urine in the form of urolithin B glucuronide after absorption of ellagitannins-containing food such as pomegranate, strawberries, red raspberries, walnuts or oak-aged red wine.

Urolithin A is a metabolite compound resulting from the transformation of ellagitannins by the gut bacteria. It belongs to the class of organic compounds known as benzo-coumarins or dibenzo-α-pyrones. Its precursors – ellagic acids and ellagitannins – are ubiquitous in nature, including edible plants, such as pomegranates, strawberries, raspberries, and walnuts. Since the 2000s, urolithin A has been subject of preliminary studies regarding its possible biological effects.

Tellimagrandin I is an ellagitannin found in plants, such as Cornus canadensis, Eucalyptus globulus, Melaleuca styphelioides, Rosa rugosa, and walnut. It is composed of two galloyl and one hexahydroxydiphenyl groups bound to a glucose residue. It differs from Tellimagrandin II only by a hydroxyl group instead of a third galloyl group. It is also structurally similar to punigluconin and pedunculagin, two more ellagitannin monomers.