 | |
 | |
| Names | |
|---|---|
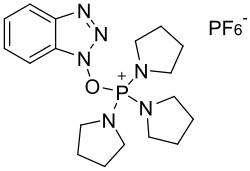
| IUPAC name (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate | |
| Other names PyBOP | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.125.168 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C18H28F6N6OP2 | |
| Molar mass | 520.401 g·mol−1 |
| Appearance | White crystals |
| Melting point | 150 °C (302 °F; 423 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant |
| GHS labelling: [1] [2] [ citation needed ] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
PyBOP (benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate) is a reagent used to prepare amides from carboxylic acids and amines in the context of peptide synthesis. [3] It can be prepared from 1-hydroxybenzotriazole and a chlorophosphonium reagent under basic conditions. [4] It is a substitute for the BOP reagent that avoids the formation of the carcinogenic waste product HMPA. [5] Thermal hazard analysis by differential scanning calorimetry (DSC) shows PyBOP is potentially explosive. [6]