
Anthocyanidins are common plant pigments, the sugar-free counterparts of anthocyanins. They are based on the flavylium cation, an oxonium ion, with various groups substituted for its hydrogen atoms. They generally change color from red through purple, blue, and bluish green as a function of pH.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is an intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, chokeberry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. Cyanidin has been found to be a potent sirtuin 6 (SIRT6) activator.

Paeonia lactiflora is a species of herbaceous perennial flowering plant in the family Paeoniaceae, native to central and eastern Asia from eastern Tibet across northern China to eastern Siberia.

African blue basil is a hybrid basil variety, a cross between camphor basil and dark opal basil. It is one of a few types of basil that are perennial. African blue basil plants are sterile, unable to produce seeds of their own, and can only be propagated by cuttings.

Dark opal basil is a cultivar of Ocimum basilicum, developed by John Scarchuk and Joseph Lent at the University of Connecticut in the 1950s. With deep purple, sometimes mottled leaves, it is grown as much for its decorative appeal as for its culinary value. Dark opal basil was a 1962 winner of the All-American Selection award.

Hibiscus schizopetalus is a species of Hibiscus native to tropical eastern Africa in Kenya, Tanzania and Mozambique. Its common names include fringed rosemallow, Japanese lantern, coral hibiscus, and spider hibiscus.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Aristotelia chilensis, known as maqui or Chilean wineberry, is a tree species in the Elaeocarpaceae family native to South America in the Valdivian temperate rainforests of Chile and adjacent regions of southern Argentina. Limited numbers of these trees are cultivated in gardens for their small fruits. Wild-harvested fruits are commercially marketed.

Purple corn or purple maize is group of flint maize varieties originating in South America, descended from a common ancestral variety termed "k'culli" in Quechua. It is most commonly grown in the Andes of Peru, Bolivia and Ecuador.
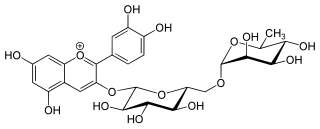
Antirrhinin is an anthocyanin. It is the 3-rutinoside of cyanidin.

Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin.

Quintinia is a genus of about 25 evergreen trees and shrubs native to the Philippines, New Guinea, New Zealand, New Caledonia, Vanuatu and Australia. Plants have alternate leaves. White or lilac flowers form at the end of stalks or on leaf axils. The fruiting body is a capsule, usually containing a large number of tiny seeds. The genus is named after the gardener Jean-Baptiste de la Quintinie.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).
Cyanidin-3-O-glucoside 2-O-glucuronosyltransferase is an enzyme with systematic name UDP-D-glucuronate:cyanidin-3-O-beta-D-glucoside 2-O-beta-D-glucuronosyltransferase. This enzyme catalyses the following chemical reaction

Cyanidin-3,5-O-diglucoside, also known as cyanin, is an anthocyanin. It is the 3,5-O-diglucoside of cyanidin.

Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.
Purple sweet potato color (PSPC) is a natural anthocyanin food coloring obtained from the sweet potato. Some cultivars, like the Ayamurasaki, released in Japan in 1995, are specially developed to have a higher anthocyanin content.

















