
Reelin, encoded by the RELN gene, is a large secreted extracellular matrix glycoprotein that helps regulate processes of neuronal migration and positioning in the developing brain by controlling cell–cell interactions. Besides this important role in early development, reelin continues to work in the adult brain. It modulates synaptic plasticity by enhancing the induction and maintenance of long-term potentiation. It also stimulates dendrite and dendritic spine development and regulates the continuing migration of neuroblasts generated in adult neurogenesis sites like the subventricular and subgranular zones. It is found not only in the brain but also in the liver, thyroid gland, adrenal gland, fallopian tube, breast and in comparatively lower levels across a range of anatomical regions.
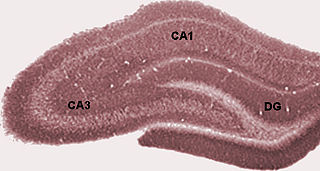
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning.

In neuroanatomy, thalamocortical radiations, also known as thalamocortical fibers, are the efferent fibers that project from the thalamus to distinct areas of the cerebral cortex. They form fiber bundles that emerge from the lateral surface of the thalamus.

The Disabled-1 (Dab1) gene encodes a key regulator of Reelin signaling. Reelin is a large glycoprotein secreted by neurons of the developing brain, particularly Cajal-Retzius cells. DAB1 functions downstream of Reln in a signaling pathway that controls cell positioning in the developing brain and during adult neurogenesis. It docks to the intracellular part of the Reelin very low density lipoprotein receptor (VLDLR) and apoE receptor type 2 (ApoER2) and becomes tyrosine-phosphorylated following binding of Reelin to cortical neurons. In mice, mutations of Dab1 and Reelin generate identical phenotypes. In humans, Reelin mutations are associated with brain malformations and mental retardation. In mice, Dab1 mutation results in the scrambler mouse phenotype.

The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane lipoprotein receptor of the low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable homology with the members of this lineage. Discovered in 1992 by T. Yamamoto, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apolipoprotein E-containing triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the VLDLR gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis.
The stratum lucidum of the hippocampus is a layer of the hippocampus between the stratum pyramidale and the stratum radiatum. It is the tract of the mossy fiber projections, both inhibitory and excitatory from the granule cells of the dentate gyrus. One mossy fiber may make up to 37 connections to a single pyramidal cell, and innervate around 12 pyramidal cells on top of that. Any given pyramidal cell in the stratum lucidum may get input from as many as 50 granule cells.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance.

Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the LRP8 gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases.

Norman–Roberts syndrome is a rare form of microlissencephaly caused by a mutation in the RELN gene. A small number of cases have been described. The syndrome was first reported by Margaret Grace Norman and M. Roberts et al. in 1976.

Homeobox protein EMX1 is a protein that in humans is encoded by the EMX1 gene. The transcribed EMX1 gene is a member of the EMX family of transcription factors. The EMX1 gene, along with its family members, are expressed in the developing cerebrum. EMX1 plays a role in specification of positional identity, the proliferation of neural stem cells, differentiation of layer-specific neuronal phenotypes and commitment to a neuronal or glial cell fate.
The trisynaptic circuit or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The trisynaptic circuit is a neural circuit in the hippocampus, which is made up of three major cell groups: granule cells in the dentate gyrus, pyramidal neurons in CA3, and pyramidal neurons in CA1. The hippocampal relay involves 3 main regions within the hippocampus which are classified according to their cell type and projection fibers. The first projection of the hippocampus occurs between the entorhinal cortex (EC) and the dentate gyrus (DG). The entorhinal cortex transmits its signals from the parahippocampal gyrus to the dentate gyrus via granule cell fibers known collectively as the perforant path. The dentate gyrus then synapses on pyramidal cells in CA3 via mossy cell fibers. CA3 then fires to CA1 via Schaffer collaterals which synapse in the subiculum and are carried out through the fornix. Collectively the dentate gyrus, CA1 and CA3 of the hippocampus compose the trisynaptic loop.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In the human and other primates, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.
Scrambler is a spontaneous mouse mutant lacking a functional DAB1 gene, resulting in a phenotype resembling that seen in the reeler mouse. The strain was first described by Sweet et al. in 1996.

Cerebellar granule cells form the thick granular layer of the cerebellar cortex and are among the smallest neurons in the brain. Cerebellar granule cells are also the most numerous neurons in the brain: in humans, estimates of their total number average around 50 billion, which means that they constitute about 3/4 of the brain's neurons.
The development of the cerebral cortex, known as corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Granule cell dispersion is one of the abnormal structural changes that has been shown in brains of patients with temporal lobe epilepsy. It has also been shown in different animal models, such as the kainic acid model, pilocarpine model, and kindling model. But granule cell dispersion was not found by using perforant pathway stimulation.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cell types in the marginal zone/layer I of the developing cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.

ATP/GTP binding protein 1 is gene that encodes the protein known as cytosolic carboxypeptidase 1 (CCP1), originally named NNA1. Mice with a naturally occurring mutation of the Agtpbp1 gene are known as pcd mice.
The supramammillary nucleus (SuM), or supramammillary area, is a thin layer of cells in the brain that lies above the mammillary bodies. It can be considered part of the hypothalamus and diencephalon. The nucleus can be divided into medial and lateral sections. The medial SuM, or SuMM, is made of smaller cells which release dopamine and give input to the lateral septal nucleus. The lateral SuM, or SuML, is made of larger cells that project to the hippocampus.















