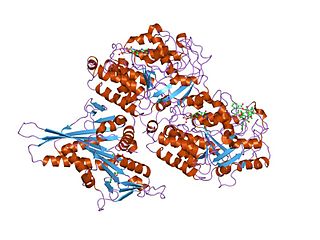
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

Podophyllotoxin (PPT) is the active ingredient in Podofilox, which is a medical cream that is used to treat genital warts and molluscum contagiosum. It is not recommended in HPV infections without external warts. It can be applied either by a healthcare provider or the person themselves.

Rhizopus oligosporus is a fungus of the family Mucoraceae and is a widely used starter culture for the production of tempeh at home and industrially. As the mold grows it produces fluffy, white mycelia, binding the beans together to create an edible "cake" of partly catabolized soybeans. The domestication of the microbe is thought to have occurred in Indonesia several centuries ago.

Combretastatin is a dihydrostilbenoid found in Combretum caffrum.

Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.
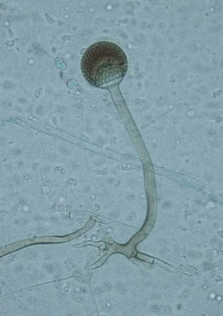
Rhizopus microsporus is a fungal plant pathogen infecting maize, sunflower, and rice.

A mitotic inhibitor, microtubule inhibitor, or tubulin inhibitor, is a drug that inhibits mitosis, or cell division, and is used in treating cancer, gout, and nail fungus. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow through continuous division that eventually spread through the body (metastasize). Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.

Eribulin, sold under the brand name Halaven among others, is an anti-cancer medication used to treat breast cancer and liposarcoma.

Maitansine (INN), or maytansine (USAN), is a cytotoxic agent. It inhibits the assembly of microtubules by binding to tubulin at the rhizoxin binding site.

Halichondrin B is a polyether macrolide originally isolated from the marine sponge Halichondria okadai by Hirata and Uemura in 1986. In the same report, these authors also reported the exquisite anticancer activity of halichondrin B against murine cancer cells both in culture and in in vivo studies. Halichondrin B was highly prioritized for development as a novel anticancer therapeutic by the United States National Cancer Institute and, in 1991, was the original test case for identification of mechanism of action by NCI's then-brand-new "60-cell line screen" The complete chemical synthesis of halichondrin B was achieved by Yoshito Kishi and colleagues at Harvard University in 1992, an achievement that ultimately enabled the discovery and development of the structurally simplified and pharmaceutically optimized analog eribulin. Eribulin was approved by the U.S. Food and Drug Administration on November 15, 2010, to treat patients with metastatic breast cancer who have received at least two prior chemotherapy regimens for late-stage disease, including both anthracycline- and taxane-based chemotherapies. Eribulin is marketed by Eisai Co. under the tradename Halaven.

Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.
Paraburkholderia endofungorum is a gram-negative, catalase and oxidase-positive, motile bacterium which is able to grow under aerobic and microaerophilic conditions without a CO2 atmosphere, from the genus Paraburkholderia and the family Burkholderiaceae.
Paraburkholderia rhizoxinica is a gram-negative, oxidase and catalase-positive, motile bacterium from the genus Paraburkholderia and the family Burkholderiaceae which was isolated from the plant pathogenic fungus, Rhizopus microsporus. The complete genome of Paraburkholderia rhizoxinica is sequenced.

Lavendamycin is a naturally occurring chemical compound discovered in fermentation broth of the soil bacterium Streptomyces lavendulae. Lavendamycin has antibiotic properties and anti-proliferative effects against several cancer cell lines. The use of lavendamycin as a cytotoxic agent in cancer therapy failed due to poor water solubility and non-specific cytotoxicity. The study of lavendamycin-based analogs designed to overcome these liabilities has been an area of research.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
Fungal-bacterial endosymbiosis encompasses the mutualistic relationship between a fungus and intracellular bacteria species residing within the fungus. Many examples of endosymbiotic relationships between bacteria and plants, algae and insects exist and have been well characterized, however fungal-bacteria endosymbiosis has been less well described.
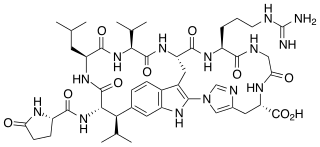
Moroidin is a biologically active compound found in the plants Dendrocnide moroides and Celosia argentea. It is a peptide composed of eight amino acids, with unusual leucine-tryptophan and tryptophan-histidine cross-links that form its two rings. Moroidin has been shown to be at least one of several bioactive compounds responsible for the painful sting of the Dendrocnide moroides plant. It also has demonstrated anti-mitotic properties, specifically by inhibition of tubulin polymerization. Anti-mitotic activity gives moroidin potential as a chemotherapy drug, and this property combined with its unusual chemical structure has made it a target for organic synthesis.
Gladiolin is a polyketide natural product produced by Burkholderia gladioli BCC0238 which is isolated from sputum of cystic fibrosis patients. It was found to be a novel macrolide antibiotic which presented an activity against Mycobacterium tuberculosis. Gladiolin is structurally much more stable than its analogue etnangien as an efficient myxobacterial RNA polymerase inhibitor due to the lack of highly labile hexaene moiety in gladiolin. The good activity and high stability of gladiolin offers it the potential for further development as an antibiotic against antibiotic-resistant M. tuberculosis.

Mucoromycota is a division within the kingdom fungi. It includes a diverse group of various molds, including the common bread molds Mucor and Rhizopus. It is a sister phylum to Dikarya.

Disorazol, a cyclic polyketide synthesized by the bacterium Sorangium cellulosum So ce12, was first detected and isolated in 1994. Its chemical structure consists of a macrocyclic ring and two oxazole rings. Disorazol A has been demonstrated to exhibit anti-fungi activities, but it was not active against yeasts. In addition, this substance demonstrates potent anti-cancer characteristics at exceptionally low picomolar levels by obstructing the mechanism of tubulin assembly and triggering the disruption of microtubules. As a result, these impacts lead to the initiation of cell apoptosis. However, disorazols cannot be directly used as drugs in the clinic due to its extremely high cytotoxicity and instability. Thus, chemical and biosynthetic synthesis pathways were designed to synthesize unnatural derivatives of disorazol in hope of reducing its cytotoxicity without decreasing its anti-cancer potency.















