Related Research Articles
A mitogen-activated protein kinase is a type of protein kinase that is specific to the amino acids serine and threonine. MAPKs are involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.
The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.

Platelet-derived growth factor receptors (PDGF-R) are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor (PDGF) family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including cancer. There are two forms of the PDGF-R, alpha and beta each encoded by a different gene. Depending on which growth factor is bound, PDGF-R homo- or heterodimerizes.

Growth factor receptor-bound protein 2, also known as Grb2, is an adaptor protein involved in signal transduction/cell communication. In humans, the GRB2 protein is encoded by the GRB2 gene.
In molecular biology, extracellular signal-regulated kinases (ERKs) or classical MAP kinases are widely expressed protein kinase intracellular signalling molecules that are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli, including growth factors, cytokines, virus infection, ligands for heterotrimeric G protein-coupled receptors, transforming agents, and carcinogens, activate the ERK pathway.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.
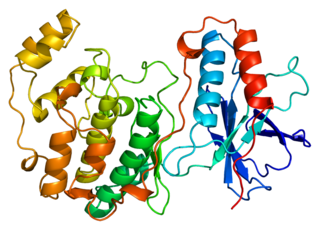
Mitogen-activated protein kinase 14, also called p38-α, is an enzyme that in humans is encoded by the MAPK14 gene.

Dual specificity protein phosphatase 1 is an enzyme that in humans is encoded by the DUSP1 gene.

Sprouty homolog 2 (Drosophila), also known as SPRY2, is a protein which in humans is encoded by the SPRY2 gene.

Fibroblast growth factor receptor substrate 2 is a protein that in humans is encoded by the FRS2 gene.

Protein TFG is a protein that in humans is encoded by the TFG gene.

Sprouty-related, EVH1 domain-containing protein 2 is a protein that in humans is encoded by the SPRED2 gene.

Protein sprouty homolog 1 is a protein that in humans is encoded by the SPRY1 gene.
Protein sprouty homolog 3 is a protein that in humans is encoded by the SPRY3 gene.
In molecular biology, the protein Sprouty is a developmental protein involved in cell signalling. It works by inhibiting the MAPK/ERK pathway.

Sprouty-related, EVH1 domain-containing protein 3 also known as Spread-3 is a protein that in humans is encoded by the SPRED3 gene.

Tyrosine phosphorylation is the addition of a phosphate (PO43−) group to the amino acid tyrosine on a protein. It is one of the main types of protein phosphorylation. This transfer is made possible through enzymes called tyrosine kinases. Tyrosine phosphorylation is a key step in signal transduction and the regulation of enzymatic activity.
References
- ↑ Zhang S, Lin Y, Itäranta P, Yagi A, Vainio S (December 2001). "Expression of Sprouty genes 1, 2 and 4 during mouse organogenesis". Mechanisms of Development. 109 (2): 367–70. doi:10.1016/S0925-4773(01)00526-3. PMID 11731251. S2CID 17189370.
- 1 2 3 Lim J, Yusoff P, Wong ES, Chandramouli S, Lao DH, Fong CW, Guy GR (November 2002). "The cysteine-rich sprouty translocation domain targets mitogen-activated protein kinase inhibitory proteins to phosphatidylinositol 4,5-bisphosphate in plasma membranes". Molecular and Cellular Biology. 22 (22): 7953–66. doi:10.1128/MCB.22.22.7953-7966.2002. PMC 134720 . PMID 12391162.
- 1 2 King JA, Straffon AF, D'Abaco GM, Poon CL, I ST, Smith CM, Buchert M, Corcoran NM, Hall NE, Callus BA, Sarcevic B, Martin D, Lock P, Hovens CM (June 2005). "Distinct requirements for the Sprouty domain for functional activity of Spred proteins". The Biochemical Journal. 388 (Pt 2): 445–54. doi:10.1042/BJ20041284. PMC 1138951 . PMID 15683364.
- ↑ Lim J, Wong ES, Ong SH, Yusoff P, Low BC, Guy GR (October 2000). "Sprouty proteins are targeted to membrane ruffles upon growth factor receptor tyrosine kinase activation. Identification of a novel translocation domain". The Journal of Biological Chemistry. 275 (42): 32837–45. doi: 10.1074/jbc.M002156200 . PMID 10887178.
- ↑ Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A (August 2001). "Spred is a Sprouty-related suppressor of Ras signalling". Nature. 412 (6847): 647–51. Bibcode:2001Natur.412..647W. doi:10.1038/35088082. PMID 11493923. S2CID 4345140.
- 1 2 Hanafusa H, Torii S, Yasunaga T, Nishida E (November 2002). "Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway". Nature Cell Biology. 4 (11): 850–8. doi:10.1038/ncb867. PMID 12402043. S2CID 31064800.