Related Research Articles

Meiosis (;from Ancient Greek μείωσις 'lessening',is a special type of cell division of germ cells in sexually-reproducing organisms that produces the gametes,the sperm or egg cells. It involves two rounds of division that ultimately result in four cells,each with only one copy of each chromosome. Additionally,prior to the division,genetic material from the paternal and maternal copies of each chromosome is crossed over,creating new combinations of code on each chromosome. Later on,during fertilisation,the haploid cells produced by meiosis from a male and a female will fuse to create a zygote,a cell with two copies of each chromosome again.

Chromosomal crossover,or crossing over,is the exchange of genetic material during sexual reproduction between two homologous chromosomes' non-sister chromatids that results in recombinant chromosomes. It is one of the final phases of genetic recombination,which occurs in the pachytene stage of prophase I of meiosis during a process called synapsis. Synapsis begins before the synaptonemal complex develops and is not completed until near the end of prophase I. Crossover usually occurs when matching regions on matching chromosomes break and then reconnect to the other chromosome.

Genetic recombination is the exchange of genetic material between different organisms which leads to production of offspring with combinations of traits that differ from those found in either parent. In eukaryotes,genetic recombination during meiosis can lead to a novel set of genetic information that can be further passed on from parents to offspring. Most recombination occurs naturally and can be classified into two types:(1) interchromosomal recombination,occurring through independent assortment of alleles whose loci are on different but homologous chromosomes;&(2) intrachromosomal recombination,occurring through crossing over.

BRCA2 and BRCA2 are human genes and their protein products,respectively. The official symbol and the official name are maintained by the HUGO Gene Nomenclature Committee. One alternative symbol,FANCD1,recognizes its association with the FANC protein complex. Orthologs,styled Brca2 and Brca2,are common in other vertebrate species. BRCA2 is a human tumor suppressor gene,found in all humans;its protein,also called by the synonym breast cancer type 2 susceptibility protein,is responsible for repairing DNA.

Memorial Sloan Kettering Cancer Center is a cancer treatment and research institution in Manhattan in New York City. MSKCC is one of 72 National Cancer Institute–designated Comprehensive Cancer Centers. Its main campus is located at 1275 York Avenue between 67th and 68th Streets in Manhattan.

Spermatocytes are a type of male gametocyte in animals. They derive from immature germ cells called spermatogonia. They are found in the testis,in a structure known as the seminiferous tubules. There are two types of spermatocytes,primary and secondary spermatocytes. Primary and secondary spermatocytes are formed through the process of spermatocytogenesis.
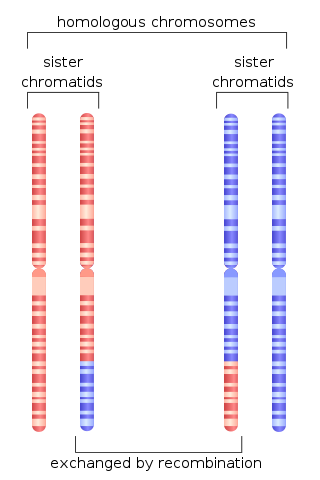
Homologous recombination is a type of genetic recombination in which genetic information is exchanged between two similar or identical molecules of double-stranded or single-stranded nucleic acids.

Spo11 is a protein that in humans is encoded by the SPO11 gene. Spo11,in a complex with mTopVIB,creates double strand breaks to initiate meiotic recombination. Its active site contains a tyrosine which ligates and dissociates with DNA to promote break formation. One Spo11 protein is involved per strand of DNA,thus two Spo11 proteins are involved in each double stranded break event.
Chromosome segregation is the process in eukaryotes by which two sister chromatids formed as a consequence of DNA replication,or paired homologous chromosomes,separate from each other and migrate to opposite poles of the nucleus. This segregation process occurs during both mitosis and meiosis. Chromosome segregation also occurs in prokaryotes. However,in contrast to eukaryotic chromosome segregation,replication and segregation are not temporally separated. Instead segregation occurs progressively following replication.

Double-strand break repair protein MRE11 is an enzyme that in humans is encoded by the MRE11 gene. The gene has been designated MRE11A to distinguish it from the pseudogene MRE11B that is nowadays named MRE11P1.

MutS protein homolog 5 is a protein that in humans is encoded by the MSH5 gene.

TRIP13 is a mammalian gene that encodes the thyroid receptor-interacting protein 13. In budding yeast,the analog for TRIP13 is PCH2. TRIP13 is a member of the AAA+ ATPase family,a family known for mechanical forces derived from ATP hydrolase reactions. The TRIP13 gene has been shown to interact with a variety of proteins and implicated in a few diseases,notably interacting with the ligand binding domain of thyroid hormone receptors,and may play a role in early-stage non-small cell lung cancer. However,recent evidence implicates TRIP13 in various cell cycle phases,including meiosis G2/Prophase and during the Spindle Assembly checkpoint (SAC). Evidence shows regulation to occur through the HORMA domains,including Hop1,Rev7,and Mad2. Of note,Mad2's involvement in the SAC is shown to be affected by TRIP13 Due to TRIP13's role in cell cycle arrest and progression,it may present opportunity as a therapeutic candidate for cancers.

Meiotic recombination protein DMC1/LIM15 homolog is a protein that in humans is encoded by the DMC1 gene.

HORMA domain-containing protein 1 (HORMAD1) also known as cancer/testis antigen 46 (CT46) is a protein that in humans is encoded by the HORMAD1 gene.

The meiotic recombination checkpoint monitors meiotic recombination during meiosis,and blocks the entry into metaphase I if recombination is not efficiently processed.
Simon N. Powell is a British cancer researcher and radiation oncologist residing in New York City.

Synthesis-dependent strand annealing (SDSA) is a major mechanism of homology-directed repair of DNA double-strand breaks (DSBs). Although many of the features of SDSA were first suggested in 1976,the double-Holliday junction model proposed in 1983 was favored by many researchers. In 1994,studies of double-strand gap repair in Drosophila were found to be incompatible with the double-Holliday junction model,leading researchers to propose a model they called synthesis-dependent strand annealing. Subsequent studies of meiotic recombination in S. cerevisiae found that non-crossover products appear earlier than double-Holliday junctions or crossover products,challenging the previous notion that both crossover and non-crossover products are produced by double-Holliday junctions and leading the authors to propose that non-crossover products are generated through SDSA.
Marcel R.M. van den Brink is a Dutch oncologist and researcher known for his research in hematopoietic stem cell transplantation for cancer patients.
Abby F. Dernburg is a professor of Cell and Developmental Biology at the University of California,Berkeley,an Investigator of the Howard Hughes Medical Institute,and a Faculty Senior Scientist at Lawrence Berkeley National Laboratory.
Maria Jasin is a developmental biologist at the Memorial Sloan Kettering Cancer Center. She is known for studying homologous recombination,a method in which double-strand breaks in DNA strands are repaired,and for discovering the role of BRCA1 and BRCA2 in cancers.
References
- ↑ "Scott Neal Keeney CV" (PDF). Retrieved June 10, 2021.
- ↑ "Scott Keeney". National Academy of Science. Retrieved June 14, 2021.
- 1 2 3 4 "At Work: Molecular Biologist Scott Keeney". Memorial Sloan Kettering Cancer Center. Retrieved June 14, 2021.
- ↑ Tontonoz, Matthew (June 21, 2018). "Out of the Closet, into the Lab: Five LGBTQ Scientists Share Their Stories". Memorial Sloan Kettering Cancer Center. Retrieved June 10, 2021.
- 1 2 "Scott Keeney Selected as a Howard Hughes Medical Institute Investigator". Memorial Sloan Kettering Cancer Center. October 1, 2008. Retrieved June 14, 2021.
- ↑ Stallard, Jim (January 6, 2017). "A Clean Break: Scientists Make Surprising Discoveries about DNA Repair". Memorial Sloan Kettering Cancer Center. Retrieved June 14, 2021.
- ↑ Claeys Bouuaert, C.; Tischfield, S. E.; Pu, S.; Mimitou, E. P.; Arias-Palomo, E.; Berger, J. M.; Keeney, S. (January 4, 2021). "Structural and functional characterization of the Spo11 core complex". Nature Structural and Molecular Biology. 28 (1): 92–102. doi:10.1038/s41594-020-00534-w. PMC 7855791 . PMID 33398171.
- ↑ "A 'Breaking' Breakthrough: Researchers at the Sloan Kettering Institute Discovery How DNA Breaks Are Controlled During Meiosis". Memorial Sloan Kettering Cancer Center. April 7, 2021. Retrieved June 14, 2021.
- ↑ "SCOTT KEENEY 2007 REGIONAL AWARD FINALIST — FACULTY". Blavatnik Awards for Young Scientists. 2007. Retrieved June 14, 2021.
- ↑ "Scott N. Keeney". American Academy of Arts and Sciences . Retrieved June 14, 2021.
- ↑ "Two Sloan Kettering Institute Scientists Elected to Esteemed National Academy of Sciences". Memorial Sloan Kettering Cancer Center. April 28, 2020. Retrieved June 14, 2021.
- 1 2 Tontonoz, Matthew. "Out of the Closet, into the Lab: Five LGBTQ Scientists Share Their Stories | Sloan Kettering Institute". www.mskcc.org. Retrieved 2021-10-08.
- ↑ "Scott Keeney". 500 Queer Scientists . Retrieved June 14, 2021.