In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐CH2‐COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to the "flexibility" caused by such a small R group. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a Clostridium tetani infection) can cause spastic paralysis due to uninhibited muscle contraction.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, with only L-cysteine being found in nature.
In organic chemistry, the Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.

An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties react to form a β-hydroxyaldehyde or β-hydroxyketone, and this is then followed by dehydration to give a conjugated enone.
Amination is the process by which an amine group is introduced into an organic molecule. This type of reaction is important because organonitrogen compounds are pervasive.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amines and is widely used in green chemistry since it can be done catalytically in one-pot under mild conditions. In biochemistry, dehydrogenase enzymes use reductive amination to produce the amino acid, glutamate. Additionally, there is ongoing research on alternative synthesis mechanisms which various metal catalysts which require more mild reaction conditions, allowing the reaction to be less energy taxing. Investigation into biocatalysts, such as EnelRED, have allowed for the reduction of chiral amines which is an important factor in pharmaceutical synthesis.
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman.
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.

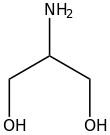
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl and amino functional groups on an alkane backbone. Most alkanolamines are colorless.
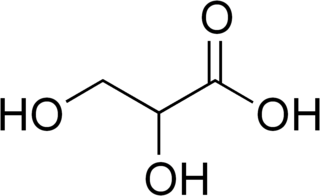
Glyceric acid refers to organic compounds with the formula HOCH2CH(OH)CO2H. It occurs naturally and is classified as three-carbon sugar acid. It is chiral. Salts and esters of glyceric acid are known as glycerates.
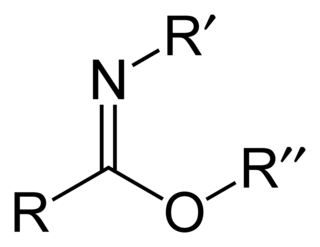
Carboximidates are organic compounds, which can be thought of as esters formed between a imidic acid and an alcohol, with the general formula R-C(=NR')OR".

Borane–tetrahydrofuran is an adduct derived from borane and tetrahydrofuran (THF). These solutions, which are colorless, are used for reductions and hydroboration, reactions that are useful in synthesis of organic compounds. The use of borane–tetrahydrofuran has been displaced by borane–dimethylsulfide, which has a longer shelf life and effects similar transformations.

In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important:

ᴅ-Amino acids are amino acids where the stereogenic carbon alpha to the amino group has the ᴅ-configuration. For most naturally-occurring amino acids, this carbon has the ʟ-configuration. ᴅ-Amino acids are occasionally found in nature as residues in proteins. They are formed from ribosomally-derived ᴅ-amino acid residues.
α-Halo carboxylic acids and esters are organic compounds with the respective formulas RCHXCO2H and RCHXCO2R' where R and R' are organic substituents. The X in these compounds is a halide, usually chloride and bromide. These compounds are often used as intermediates in the preparation of more elaborate derivatives. They are often potent alkylating agents. The mono halide derivatives are chiral.