
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit (C2H2)n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. They are white solids with the formula (C4H2S)n for the parent PT. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have substituents at the 3- or 4-position. They are also white solids, but tend to be soluble in organic solvents.
Smart materials, called also intelligent or responsive materials, are designed materials that have one or more properties that can be significantly changed in a controlled fashion by external stimuli, such as stress, moisture, electric or magnetic fields, light, temperature, pH, or chemical compounds. Smart materials are the basis of many applications, including sensors and actuators, or artificial muscles, particularly as electroactive polymers (EAPs).,,

Electrochromism is the phenomenon where the color of a material changes by applying a voltage. By doing so, an electrochromic window can block UV, visible or (near) infrared light by the push of a button. The ability to control transmittance of (near) infrared light limits the amount of energy needed to cool during summer and heat during winter. For this reason it is researched for use in smart windows to increase energy efficiency of buildings.

Poly(3,4-ethylenedioxythiophene) or PEDOT is a conducting polymer based on 3,4-ethylenedioxythiophene or EDOT. It was reported in 1991.
Ormosil is a shorthand phrase for organically modified silica or organically modified silicate. In general, ormosils are produced by adding silane to silica-derived gel during the sol-gel process. They are engineered materials that show great promise in a wide range of applications such as:
Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures that the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.
A polymer-based battery uses organic materials instead of bulk metals to form a battery. Currently accepted metal-based batteries pose many challenges due to limited resources, negative environmental impact, and the approaching limit of progress. Redox active polymers are attractive options for electrodes in batteries due to their synthetic availability, high-capacity, flexibility, light weight, low cost, and low toxicity. Recent studies have explored how to increase efficiency and reduce challenges to push polymeric active materials further towards practicality in batteries. Many types of polymers are being explored, including conductive, non-conductive, and radical polymers. Batteries with a combination of electrodes are easier to test and compare to current metal-based batteries, however batteries with both a polymer cathode and anode are also a current research focus. Polymer-based batteries, including metal/polymer electrode combinations, should be distinguished from metal-polymer batteries, such as a lithium polymer battery, which most often involve a polymeric electrolyte, as opposed to polymeric active materials.
Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks that are topologically isomorphic with zeolites. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIF’s are being investigated for applications such as carbon capture.
Bioelectronics is a field of research in the convergence of biology and electronics.

Poly(dichlorophosphazene), also called dichlorophosphazine polymer or phosphonitrilechloride polymer, is a chemical compound with formula (PNCl2)n. It is an inorganic (hence carbon-free) chloropolymer, whose backbone is a chain of alternating phosphorus and nitrogen atoms, connected by alternating single and double covalent bonds.
Harry R. Allcock is Evan Pugh Professor of chemistry at Pennsylvania State University.
Polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula occur. can be hydrogen atoms or organic substituents. If all substituents R are H atoms, the polymer is designated as Perhydropolysilazane, Polyperhydridosilazane, or Inorganic Polysilazane ([H2Si–NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes are isoelectronic with and close relatives to Polysiloxanes (silicones).
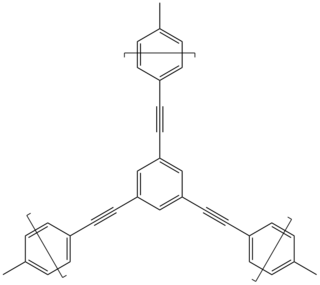
Conjugated microporous polymers (CMPs) are a sub-class of porous materials that are related to structures such as zeolites, metal-organic frameworks, and covalent organic frameworks, but are amorphous in nature, rather than crystalline. CMPs are also a sub-class of conjugated polymers and possess many of the same properties such as conductivity, mechanical rigidity, and insolubility. CMPs are created through the linking of building blocks in a π-conjugated fashion and possess 3-D networks. Conjugation extends through the system of CMPs and lends conductive properties to CMPs. Building blocks of CMPs are attractive in that the blocks possess broad diversity in the π units that can be used and allow for tuning and optimization of the skeleton and subsequently the properties of CMPs. Most building blocks have rigid components such as alkynes that cause the microporosity. CMPs have applications in gas storage, heterogeneous catalysis, light emitting, light harvesting, and electric energy storage.
Conducting polymer metal nanocomposites are.
Electronic skin refers to flexible, stretchable and self-healing electronics that are able to mimic functionalities of human or animal skin. The broad class of materials often contain sensing abilities that are intended to reproduce the capabilities of human skin to respond to environmental factors such as changes in heat and pressure.

















