Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

Potassium hexacyanidoferrate(II) is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Citric acid is an organic compound with the skeletal formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.

Sodium chloride, commonly known as edible salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chlorine ions. It is transparent or translucent, brittle, hygroscopic, and occurs as the mineral halite. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.
Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a chemical compound with a number of uses. It is the potassium acid salt of tartaric acid (a carboxylic acid). In cooking, it is known as cream of tartar.
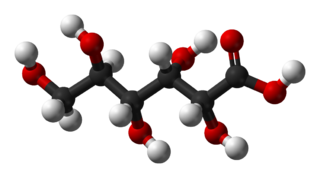
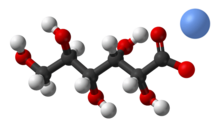
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4CO2H. A white solid, it forms the gluconate anion in neutral aqueous solution. The salts of gluconic acid are known as "gluconates". Gluconic acid, gluconate salts, and gluconate esters occur widely in nature because such species arise from the oxidation of glucose. Some drugs are injected in the form of gluconates.

Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins, but is increasingly being accomplished using nanofiltration or reverse osmosis membranes.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This salt is colorless deliquescent, and Hygroscopic.

Calcium lactate is a white crystalline salt with formula C
6H
10CaO
6, consisting of two lactate anions H
3C(CHOH)CO−
2 for each calcium cation Ca2+
. It forms several hydrates, the most common being the pentahydrate C
6H
10CaO
6·5H
2O.

Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped and stored. The anhydrous form is hygroscopic. Solutions of sodium bisulfate are acidic, with a 1M solution having a pH of slightly below 1.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration.

Calcium gluconate is the calcium salt of gluconic acid and is used as a mineral supplement and medication. As a medication it is used by injection into a vein to treat low blood calcium, high blood potassium, and magnesium toxicity. Supplementation is generally only required when there is not enough calcium in the diet. Supplementation may be done to treat or prevent osteoporosis or rickets. It can also be taken by mouth but is not recommended for injection into a muscle.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such as a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.

Nitrilotriacetonitrile (NTAN) is a precursor for nitrilotriacetic acid, for tris(2-aminoethyl)amine and for the epoxy resin crosslinker aminoethylpiperazine.
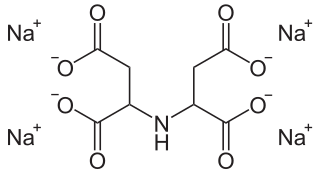
Tetrasodium iminodisuccinate is a sodium salt of iminodisuccinic acid, also referred to as N-(1,2-dicarboxyethyl)aspartic acid.

Trisodium N-(1-carboxylatoethyl)iminodiacetate, methylglycinediacetic acid trisodium salt (MGDA-Na3) or trisodium α-DL-alanine diacetate (α-ADA), is the trisodium anion of N-(1-carboxyethyl)iminodiacetic acid and a tetradentate complexing agent. It forms stable 1:1 chelate complexes with cations having a charge number of at least +2, e.g. the "hard water forming" cations Ca2+ or Mg2+. α-ADA is distinguished from the isomeric β-alaninediacetic acid by better biodegradability and therefore improved environmental compatibility.