The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
IARC group 3 substances, chemical mixtures and exposure circumstances are those that can not be classified in regard to their carcinogenicity to humans by the International Agency for Research on Cancer (IARC). This category is used most commonly for agents, mixtures and exposure circumstances for which the level of evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans, but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Biancaea sappan is a species of flowering tree in the legume family, Fabaceae, that is native to tropical Asia. Common names in English include sappanwood and Indian redwood. It was previously ascribed to the genus Caesalpinia. Sappanwood is related to brazilwood, and was itself called brasilwood in the Middle Ages.
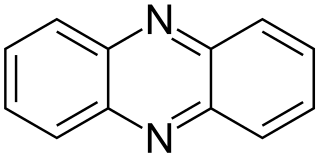
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution.
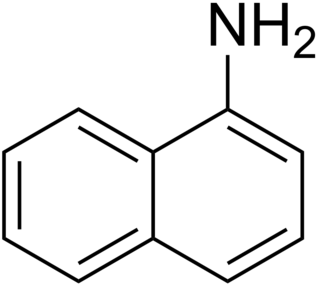
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.

Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant, for which it is named, as well as in the common walnut and water hyacinth. Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via a Michael addition reaction, resulting in a strong permanent stain that lasts until the skin or hair is shed. Darker colored staining is due to more lawsone–keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning agents and sunscreens. Lawsone is a 1,4-naphthoquinone derivative, an analog of hydroxyquinone containing one additional ring.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.

Tetrahydroxy-1,4-benzoquinone, also called tetrahydroxy-p-benzoquinone, tetrahydroxybenzoquinone, or tetrahydroxyquinone, is an organic compound with formula C6O2(OH)4. Its molecular structure consists of a cyclohexadiene ring with four hydroxyl groups and two ketone groups in opposite (para) positions.

Spinochrome E is a polyhydroxylated 1,4-naphthoquinone pigment found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin commonly known as spinochromes. These natural phenolic compounds are quinones with potential pharmacological properties. The several hydroxyl groups are appropriate for free-radical scavenging, which diminishes ROS and prevents redox imbalance. Mechanisms are described such as scavenging of reactive oxygen species (ROS), interaction with lipid peroxide radicals, chelation of metal ions, inhibition of lipid peroxidation and regulation of the cell redox potential.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
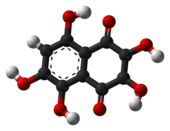
Spinochrome B (2,3,5,7-tetrahydroxynaphthoquinone) is an organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through the replacement of four hydrogen atoms by hydroxyl (OH) groups.
A dihydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of naphthoquinone through replacement of two hydrogen atoms (H) by hydroxyl groups (OH).
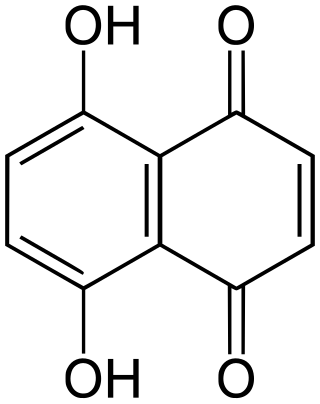
Naphthazarin, often called 5,8-dihydroxy-1,4-naphthoquinone or 5,8-dihydroxy-1,4-naphthalenedione (IUPAC), is a naturally occurring organic compound with formula C
10H
6O
4, formally derived from 1,4-naphthoquinone through replacement of two hydrogen atoms by hydroxyl (OH) groups. It is thus one of many dihydroxynaphthoquinone structural isomers.
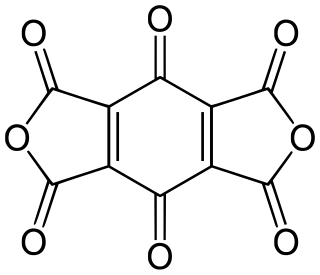
Benzoquinonetetracarboxylic dianhydride is an organic compound with formula C
10O
8 which can be seen as the result of removing two molecules of water H
2O from benzoquinonetetracarboxylic acid.

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.
The enzyme 1,4-dihydroxy-2-naphthoyl-CoA hydrolase (EC 3.1.2.28; systematic name 1,4-dihydroxy-2-naphthoyl-CoA hydrolase) catalyses the following reaction:

Chika Kuroda was a Japanese chemist whose research focused on natural pigments. She was the first woman in Japan to receive a Bachelor of Science.
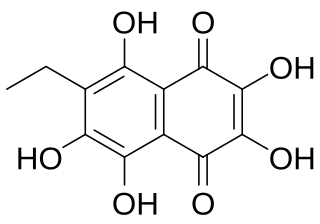
Echinochrome A (7-ethyl-2,3,5,6,8-pentahydroxy-1,4-naphthoquinone) is a polyhydroxylated 1,4-naphthoquinone, a type of pigments commonly found in sea urchin shell ("test"), spine, gonads, coelomic fluid, and eggs, of sea urchin. These type of pigments are commonly known as spinochromes and are natural marine phenolic compounds with potential pharmacological effects and modes of action.