Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often requires a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful.

A natural product is a chemical compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.

Lupinine is a quinolizidine alkaloid present in the genus Lupinus of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of Lupinus plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of Lupinus.
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. It contains a five-membered ring consisting of four carbon atoms and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.

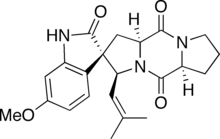
Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
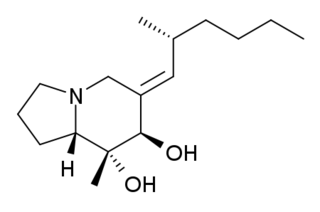
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.

Yuehchukene is a dimeric indole alkaloid natural product that possesses anti-fertility and estrogenic activities. Yuehchukene is isolated from the roots of Murraya paniculata and other species of the plant genus Murraya. Its natural abundance is in the range of 10-52 ppm.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.

Indole is an aromatic heterocyclic organic compound with formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
2,5-Diketopiperazine, also known as piperazine-2,5-dione and as the cyclodipeptide cyclo(Gly-Gly), is an organic compound and the smallest cyclic dipeptide that consists of a six-membered ring containing two amide linkages where the two nitrogen atoms and the two carbonyls are at opposite positions in the ring. It was first synthesized by Curtius and Gloebel in 1888 and was the first compound containing a peptide bond to be studied by X-ray crystallography in 1938. It occurs in cocoa and bread and has a metallic and bitter taste.

(−)-Aurantiamine is a blue fluorescence metabolite produced by the fungus Penicillium aurantiogriseum, the most common fungi found in cereals. (−)-Aurantiamine belongs to a class of naturally occurring 2,5-diketopiperazines featuring a dehydrohistidine residue that exhibit important biological activities, such as anti-cancer or neurotoxic effects. It is the isopropyl analog of the microtubule binding agent (−)-phenylahistin but is 40 times less active than the latter on P388 cell proliferation. The total asymmetric synthesis of (−)-aurantiamine has been described.
Fellutanine A, B, C and D are bio-active diketopiperazine alkaloids isolated from the cultures of Penicillium fellutanum, that belongs to a class of naturally occurring 2,5-diketopiperazines. Originally they were thought to be based on the "trans" cyclic dipetide cyclo(L-Trp-D-Trp) but were later shown to be based on the "cis" cyclic dipetide cyclo(L-Trp-L-Trp). This was also confirmed when fellutanine A, B and C were isolated from Penicillium simplicissimum. The fellutanines A−C, are non-annulated analogues of cyclo(L-Trp-L-Trp), but unlike their diannulated analogue fellutanine D are not cytotoxic.

Verruculogen is a mycotoxin produced by certain strains of aspergillus that belongs to a class of naturally occurring 2,5-diketopiperazines. It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a Salmonella/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.
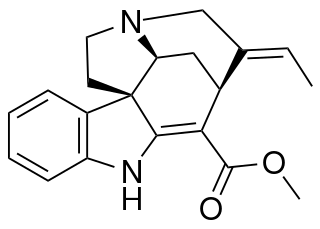
Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Gigactonine is a naturally occurring diterpene alkaloid first isolated from Aconitum gigas. It occurs widely in the Ranunculaceae plant family. The polycyclic ring system of this chemical compound contains nineteen carbon atoms and one nitrogen atom, which is the same as in aconitine and this is reflected in its preferred IUPAC name.
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.

Dideoxyverticillin A, also known as (+)-11,11′-dideoxyverticillin A, is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999. It has also been found in the marine fungus Bionectriaceae, and belongs to a class of naturally occurring 2,5-diketopiperazines.

Piperolactam A is a natural product alkaloid found in many plants and first isolated from roots of Piper longum. As a group, such compounds are called aristolactams, and are related to aristolochic acid.